Polarity Chart Of Solvents
Polarity Chart Of Solvents - The polarity of bonds mainly arises from the act between. In chemistry, polarity refers to the way in which atoms bond with each other. The quality of being opposite: When atoms come together in chemical bonding, they share electrons. See examples of polarity used. Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. Polarity in chemistry refers to the distribution of electrons in a molecule, leading to uneven distribution of charge and the development of a positive and a negative pole within the. The quality of having two poles: Polarity, in general, refers to the physical properties of compounds such as boiling point, melting points, and their solubilities. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively. Polarity, in general, refers to the physical properties of compounds such as boiling point, melting points, and their solubilities. In simple words, polarity happens when there is an uneven. See examples of polarity used. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively. The quality of being opposite: The quality of having two poles: In chemistry, polarity refers to the way in which atoms bond with each other. The polarity of bonds mainly arises from the act between. Polarity in chemistry refers to the distribution of electrons in a molecule, leading to uneven distribution of charge and the development of a positive and a negative pole within the. A polar molecule arises when. While bonds between identical atoms such as two of hydrogen are electrically uniform in. The meaning of polarity is the quality or condition inherent in a body that exhibits opposite properties or powers in opposite parts or directions or that exhibits contrasted properties or. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical. See examples of polarity used. The polarity of bonds mainly arises from the act between. Polarity in chemistry refers to the distribution of electrons in a molecule, leading to uneven distribution of charge and the development of a positive and a negative pole within the. In simple words, polarity happens when there is an uneven. The meaning of polarity is. The quality of being opposite: See examples of polarity used. The polarity of bonds mainly arises from the act between. The quality of having two poles: In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively. See examples of polarity used. The quality of having two poles: Polarity refers to the condition in which the electric charges on a molecule are separated, leading to a partial positive charge at one end and a partial negative charge at the other. In simple words, polarity happens when there is an uneven. The property or characteristic that produces unequal. Polarity refers to the existence of two opposite charges or poles within a system — like positive and negative charges. Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. The meaning of polarity is the quality or condition inherent in a body that exhibits opposite properties or powers in opposite parts or directions. While bonds between identical atoms such as two of hydrogen are electrically uniform in. See examples of polarity used. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively. Polarity refers to the condition in which the electric charges on. In simple words, polarity happens when there is an uneven. While bonds between identical atoms such as two of hydrogen are electrically uniform in. Polarity refers to the condition in which the electric charges on a molecule are separated, leading to a partial positive charge at one end and a partial negative charge at the other. A polar molecule arises. Polarity, in general, refers to the physical properties of compounds such as boiling point, melting points, and their solubilities. The property or characteristic that produces unequal physical effects at different points in a body or system, as a magnet or storage battery. Polarity refers to the existence of two opposite charges or poles within a system — like positive and. The quality of being opposite: The property or characteristic that produces unequal physical effects at different points in a body or system, as a magnet or storage battery. The polarity of bonds mainly arises from the act between. Polarity refers to the existence of two opposite charges or poles within a system — like positive and negative charges. See examples. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively. The quality of having two poles: Polarity refers to the existence of two opposite charges or poles within a system — like positive and negative charges. The property or characteristic. The polarity of bonds mainly arises from the act between. The quality of being opposite: Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. Polarity refers to the existence of two opposite charges or poles within a system — like positive and negative charges. Polarity refers to the condition in which the electric charges on a molecule are separated, leading to a partial positive charge at one end and a partial negative charge at the other. In simple words, polarity happens when there is an uneven. Polarity in chemistry refers to the distribution of electrons in a molecule, leading to uneven distribution of charge and the development of a positive and a negative pole within the. The quality of having two poles: The property or characteristic that produces unequal physical effects at different points in a body or system, as a magnet or storage battery. Polarity, in general, refers to the physical properties of compounds such as boiling point, melting points, and their solubilities. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively. While bonds between identical atoms such as two of hydrogen are electrically uniform in. See examples of polarity used.Polarity Chart Of Solvents
Polarity Chart Of Solvents
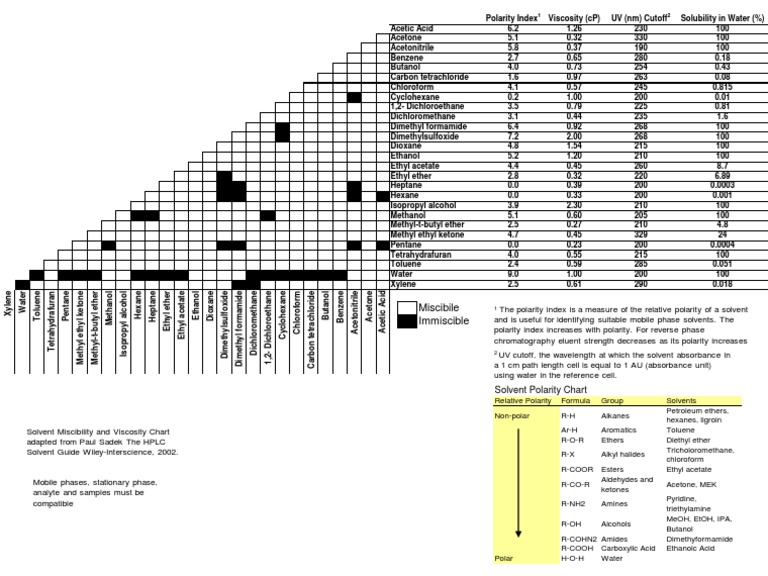
Solvent Miscibility and Polarity Chart
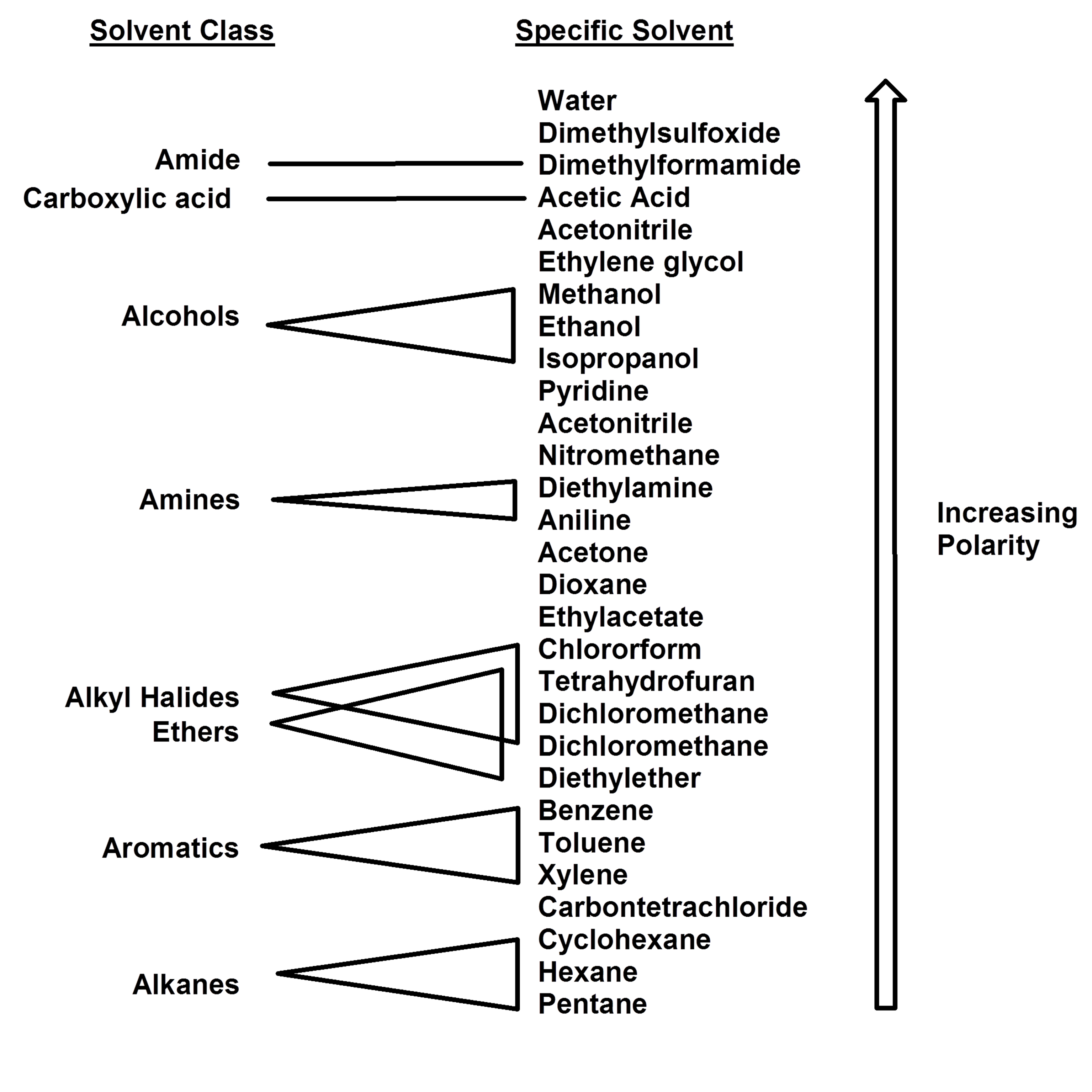
Organic Chem 15 For organic solvents, likes dissolve likes
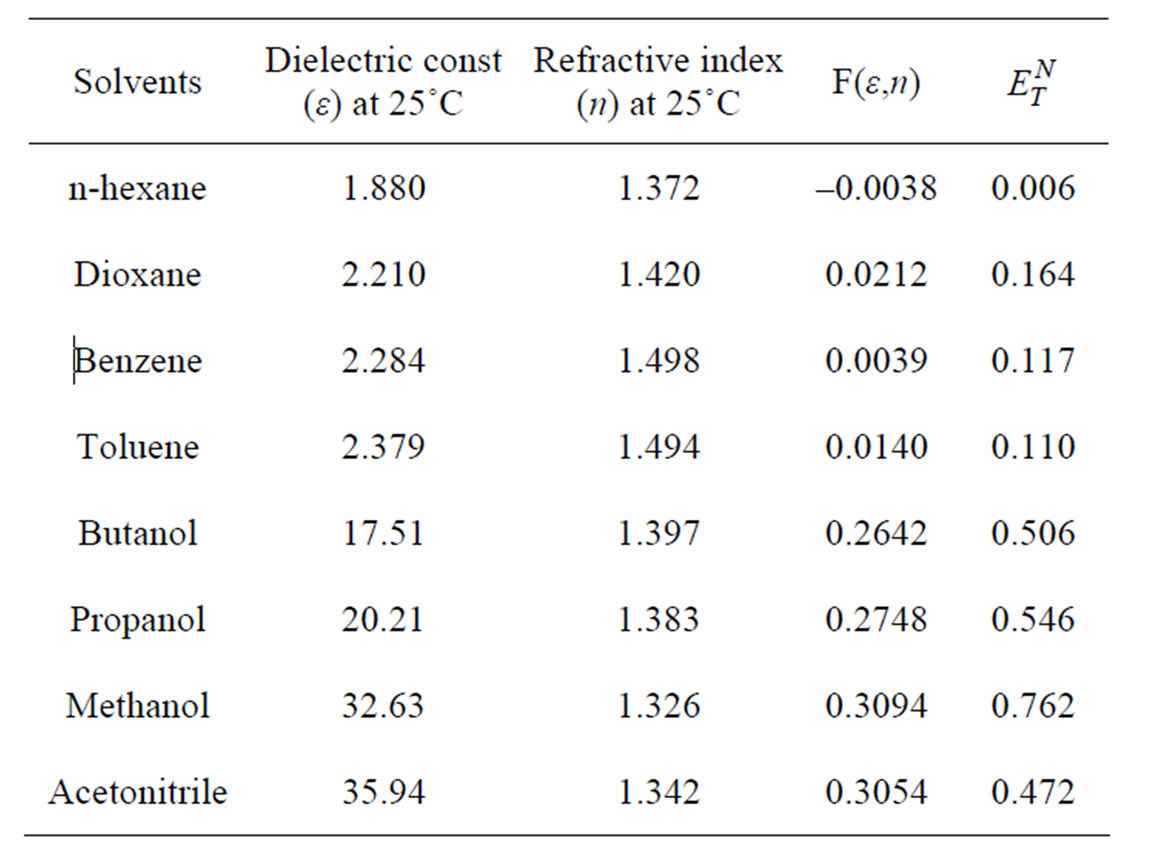
Determine Solvent Polarity Index Table
Polarity Chart Of Solvents
Solvent Polarity of Some DES Download Table
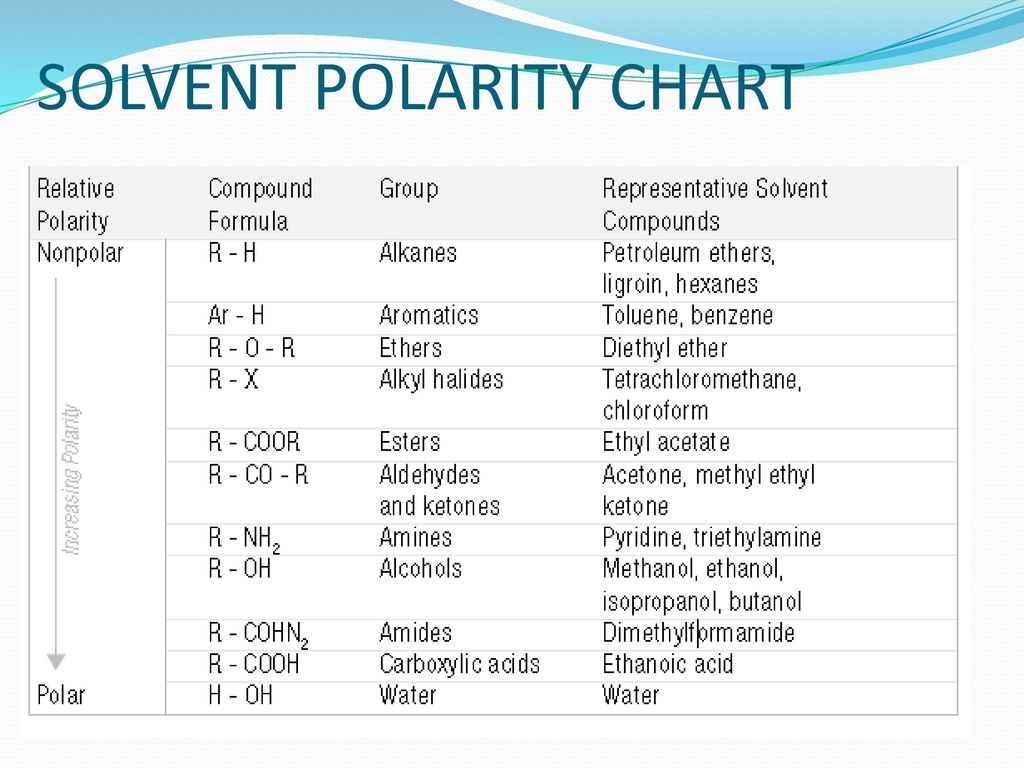
Solvent Polarity Chart Minga
Solvent Polarity Chart A Visual Reference of Charts Chart Master
Organic Solvent Polarity Chart at Rose Braddon blog
In Chemistry, Polarity Refers To The Way In Which Atoms Bond With Each Other.
When Atoms Come Together In Chemical Bonding, They Share Electrons.
A Polar Molecule Arises When.
The Meaning Of Polarity Is The Quality Or Condition Inherent In A Body That Exhibits Opposite Properties Or Powers In Opposite Parts Or Directions Or That Exhibits Contrasted Properties Or.
Related Post: