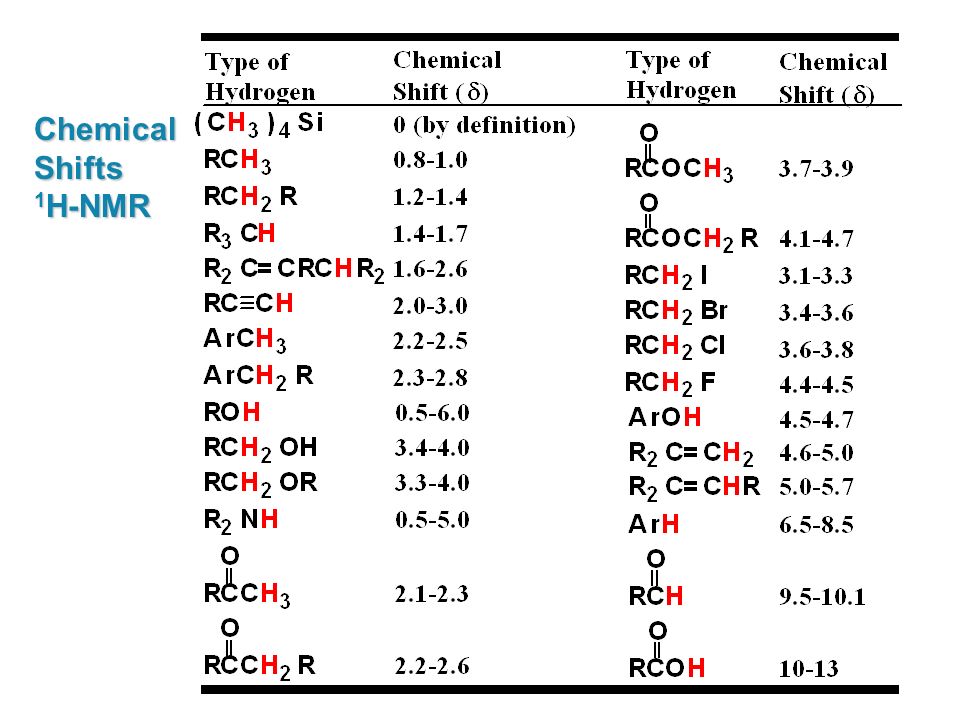
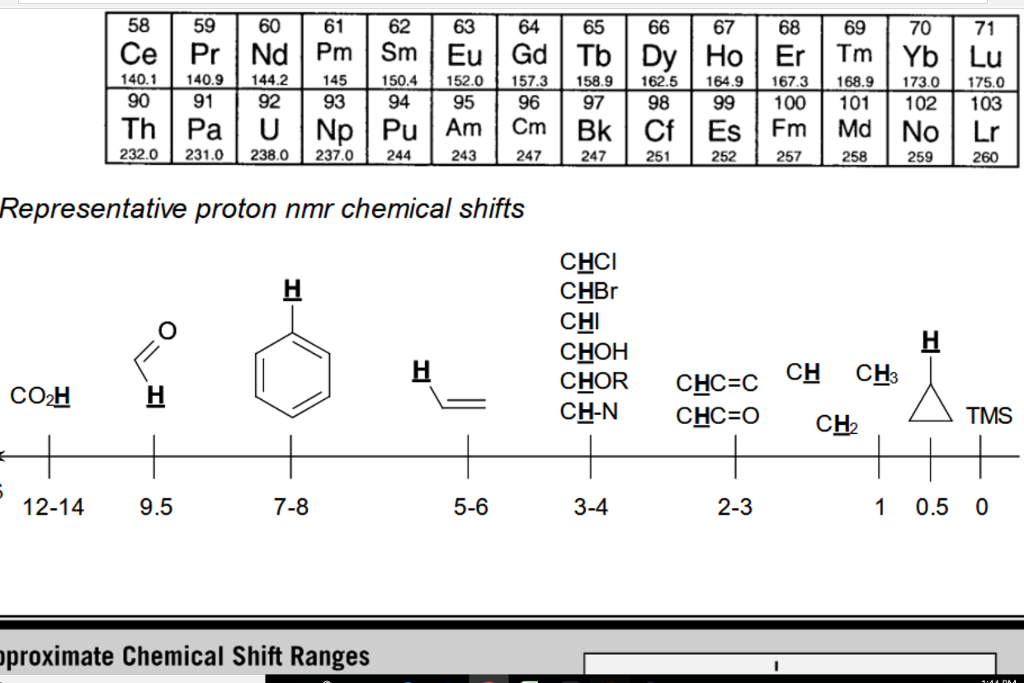
H Nmr Chart
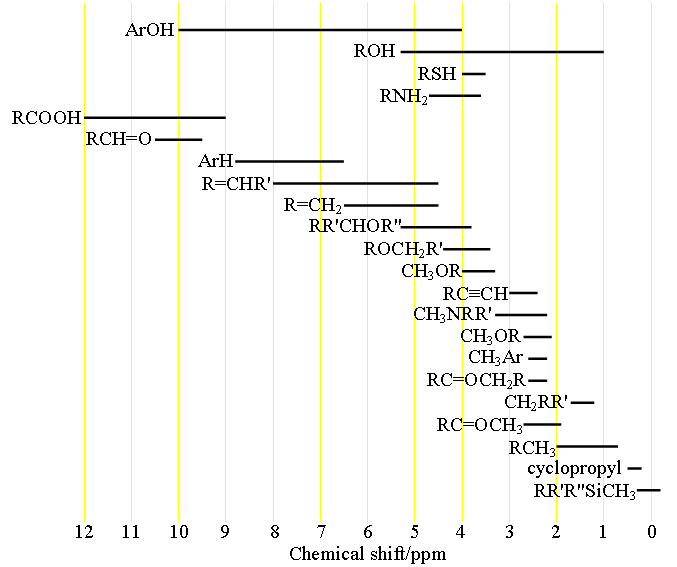
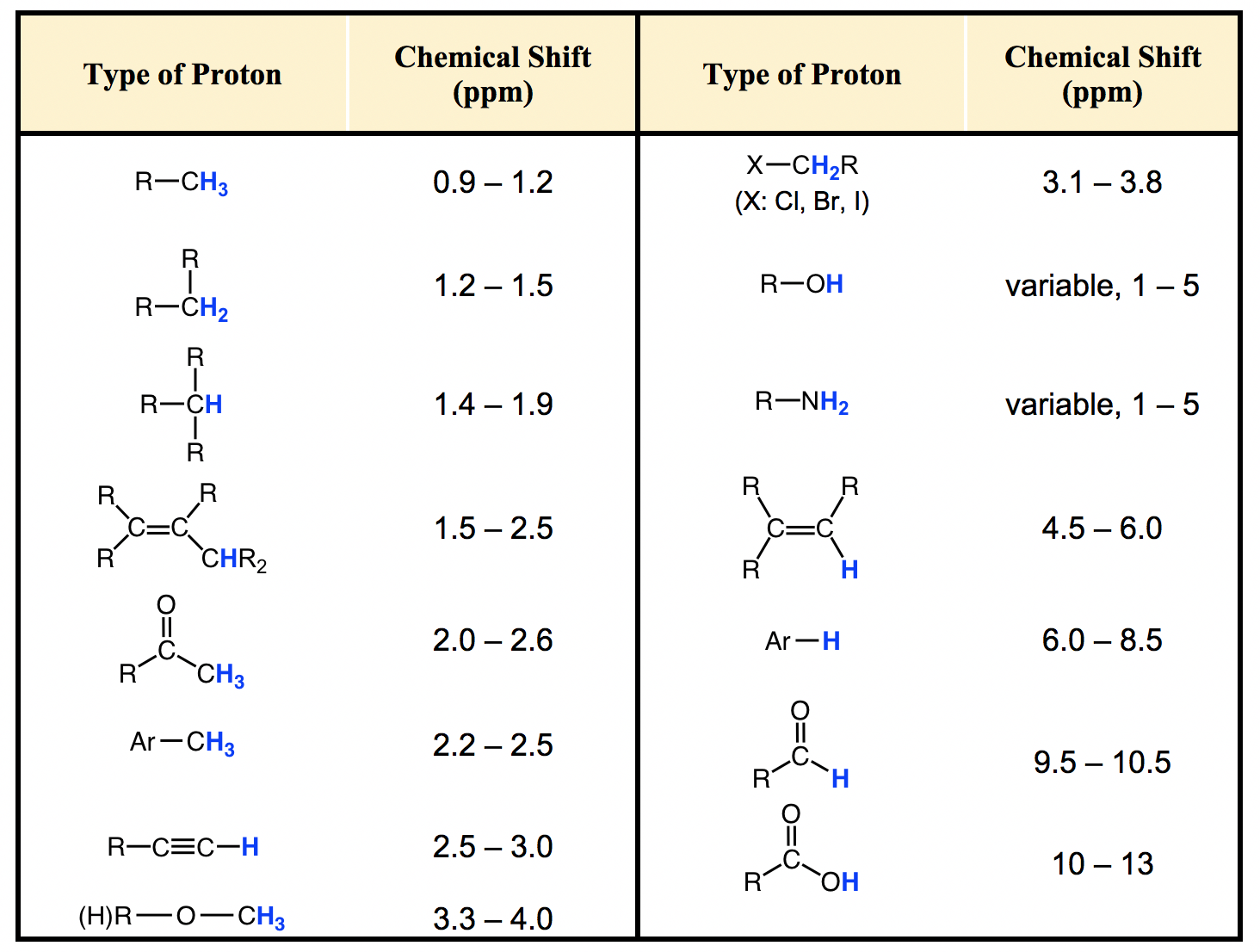
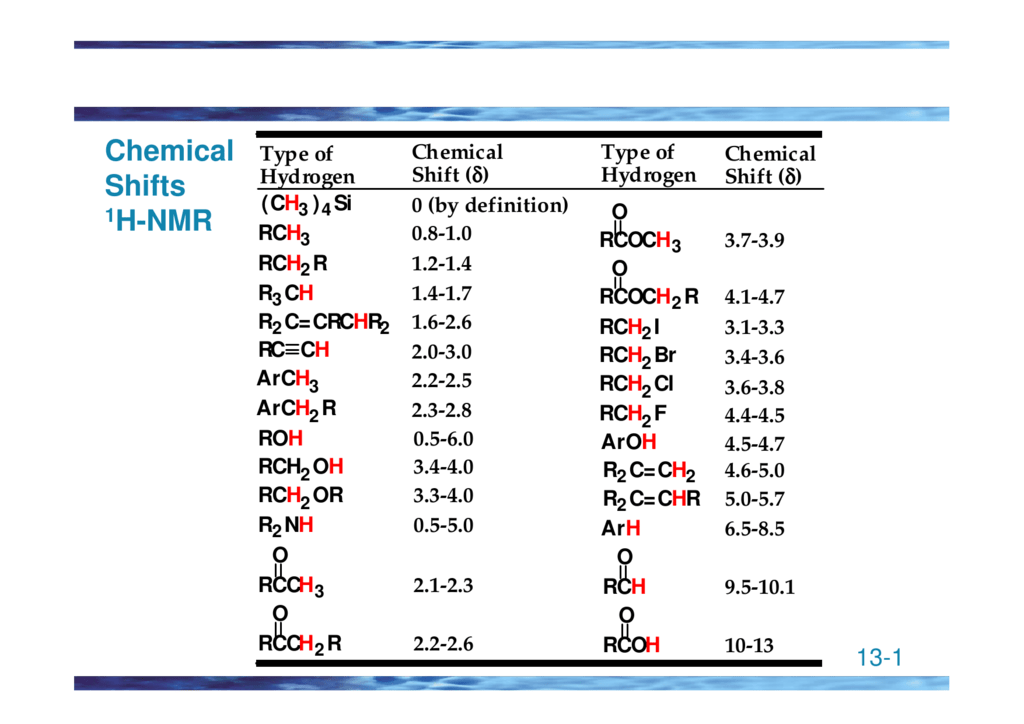
H Nmr Chart - The effect of electronegativity and magnetic anisotropy on protons in upfield and downfield regions. A guide to 1h nmr chemical shift values nuclear magnetic resonance (nmr) is a commonly used technique for organic compound structure determination. Here we present the nmr shifts of the most commonly used solvents and impurities in organic synthesis measured in the 7 most frequently used deuterated solvents. Alkene region modified from earlier handout It also includes nmr summary data on coupling constants and chemical shift of. Overview of typical 1h nmr shifts. Table of characteristic proton nmr shifts. If a protic deuterated solvent is used (e.g., d2o or cd3od), then the nh and oh protons will exchange with the deuterium and the peaks will shrink or disappear entirely, since d (2h) does. Understanding the basics of nmr theory gets us ready to move on to the most important and practical part in this section, that is how to understand the 1h nmr spectrum and elucidate the. You can download this chart as a printable acrobat pdf file. A guide to 1h nmr chemical shift values nuclear magnetic resonance (nmr) is a commonly used technique for organic compound structure determination. Understanding the basics of nmr theory gets us ready to move on to the most important and practical part in this section, that is how to understand the 1h nmr spectrum and elucidate the. From table 14.4 (labbook) or table h.6 (spec book) substituted alkanes 1. In the nmr spectrum of the dianion, the innermost methylene protons (red) give an nmr signal at +22.2 ppm, the adjacent methylene protons (blue) give a signal at +12.6 ppm, and the methyl. The effect of electronegativity and magnetic anisotropy on protons in upfield and downfield regions. If a protic deuterated solvent is used (e.g., d2o or cd3od), then the nh and oh protons will exchange with the deuterium and the peaks will shrink or disappear entirely, since d (2h) does. Overview of typical 1h nmr shifts. It describes nuclear magnetic resonance (nmr) in details relevant to organic chemistry. You can download this chart as a printable acrobat pdf file. Table of characteristic proton nmr shifts. A guide to 1h nmr chemical shift values nuclear magnetic resonance (nmr) is a commonly used technique for organic compound structure determination. Nmr chemical shift and ppm value chart. It describes nuclear magnetic resonance (nmr) in details relevant to organic chemistry. Overview of typical 1h nmr shifts. From table 14.4 (labbook) or table h.6 (spec book) substituted alkanes 1. Nmr chemical shift and ppm value chart. You can download this chart as a printable acrobat pdf file. A guide to 1h nmr chemical shift values nuclear magnetic resonance (nmr) is a commonly used technique for organic compound structure determination. Alkene region modified from earlier handout If a protic deuterated solvent is used (e.g., d2o or cd3od), then the nh. Nmr chemical shift and ppm value chart. In the nmr spectrum of the dianion, the innermost methylene protons (red) give an nmr signal at +22.2 ppm, the adjacent methylene protons (blue) give a signal at +12.6 ppm, and the methyl. Understanding the basics of nmr theory gets us ready to move on to the most important and practical part in. You can download this chart as a printable acrobat pdf file. It also includes nmr summary data on coupling constants and chemical shift of. A guide to 1h nmr chemical shift values nuclear magnetic resonance (nmr) is a commonly used technique for organic compound structure determination. Overview of typical 1h nmr shifts note: From table 14.4 (labbook) or table h.6. It describes nuclear magnetic resonance (nmr) in details relevant to organic chemistry. Understanding the basics of nmr theory gets us ready to move on to the most important and practical part in this section, that is how to understand the 1h nmr spectrum and elucidate the. A guide to 1h nmr chemical shift values nuclear magnetic resonance (nmr) is a. It describes nuclear magnetic resonance (nmr) in details relevant to organic chemistry. Alkene region modified from earlier handout A guide to 1h nmr chemical shift values nuclear magnetic resonance (nmr) is a commonly used technique for organic compound structure determination. From table 14.4 (labbook) or table h.6 (spec book) substituted alkanes 1. Table of characteristic proton nmr shifts. From table 14.4 (labbook) or table h.6 (spec book) substituted alkanes 1. Understanding the basics of nmr theory gets us ready to move on to the most important and practical part in this section, that is how to understand the 1h nmr spectrum and elucidate the. Overview of typical 1h nmr shifts note: Alkene region modified from earlier handout It. Overview of typical 1h nmr shifts. You can download this chart as a printable acrobat pdf file. Overview of typical 1h nmr shifts note: It describes nuclear magnetic resonance (nmr) in details relevant to organic chemistry. From table 14.4 (labbook) or table h.6 (spec book) substituted alkanes 1. Overview of typical 1h nmr shifts. Overview of typical 1h nmr shifts note: A guide to 1h nmr chemical shift values nuclear magnetic resonance (nmr) is a commonly used technique for organic compound structure determination. It also includes nmr summary data on coupling constants and chemical shift of. Alkene region modified from earlier handout It describes nuclear magnetic resonance (nmr) in details relevant to organic chemistry. Overview of typical 1h nmr shifts note: Alkene region modified from earlier handout Here we present the nmr shifts of the most commonly used solvents and impurities in organic synthesis measured in the 7 most frequently used deuterated solvents. The effect of electronegativity and magnetic anisotropy on protons. Nmr chemical shift and ppm value chart. The effect of electronegativity and magnetic anisotropy on protons in upfield and downfield regions. It also includes nmr summary data on coupling constants and chemical shift of. Overview of typical 1h nmr shifts note: You can download this chart as a printable acrobat pdf file. A guide to 1h nmr chemical shift values nuclear magnetic resonance (nmr) is a commonly used technique for organic compound structure determination. Here we present the nmr shifts of the most commonly used solvents and impurities in organic synthesis measured in the 7 most frequently used deuterated solvents. Table of characteristic proton nmr shifts. It describes nuclear magnetic resonance (nmr) in details relevant to organic chemistry. Alkene region modified from earlier handout Understanding the basics of nmr theory gets us ready to move on to the most important and practical part in this section, that is how to understand the 1h nmr spectrum and elucidate the. From table 14.4 (labbook) or table h.6 (spec book) substituted alkanes 1.H Nmr Spectrum Chart
H Nmr Chemical Shift Chart Ponasa
6.6 ¹H NMR Spectra and Interpretation (Part I) Organic Chemistry I
Analytical Chemistry A Guide to Proton Nuclear Resonance (NMR) Compound Interest
Nmr Shift Chart vrogue.co
H Nmr Chemical Shift Chart Ponasa
H Nmr Spectroscopy Table at Lois Coffman blog
NMR Spectroscopy Principles, Interpreting an NMR Spectrum and Common Problems Technology Networks
Nmr Values Chart
H Nmr Chemical Shift Chart Ponasa
If A Protic Deuterated Solvent Is Used (E.g., D2O Or Cd3Od), Then The Nh And Oh Protons Will Exchange With The Deuterium And The Peaks Will Shrink Or Disappear Entirely, Since D (2H) Does.
Overview Of Typical 1H Nmr Shifts.
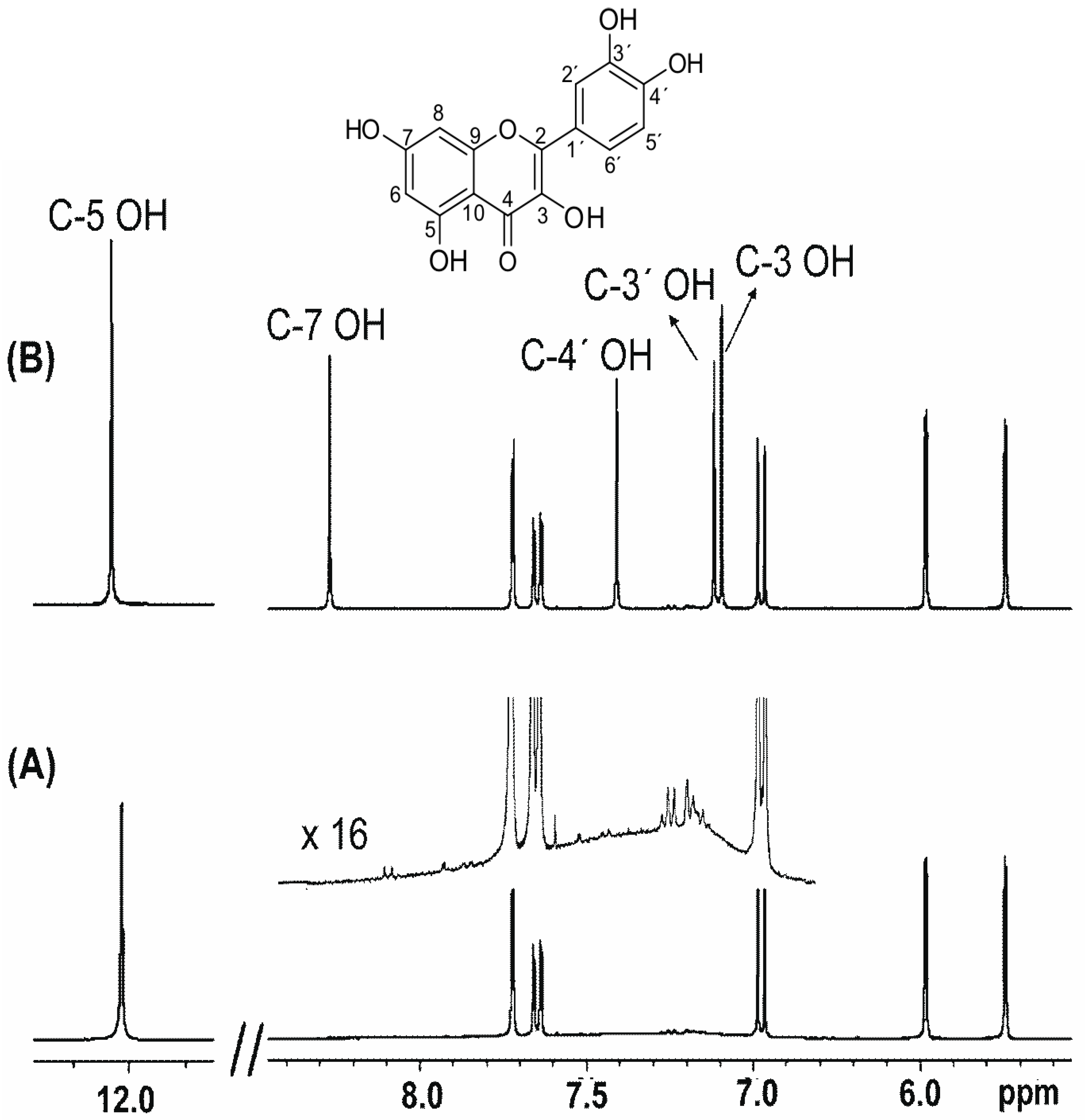
In The Nmr Spectrum Of The Dianion, The Innermost Methylene Protons (Red) Give An Nmr Signal At +22.2 Ppm, The Adjacent Methylene Protons (Blue) Give A Signal At +12.6 Ppm, And The Methyl.
Related Post: