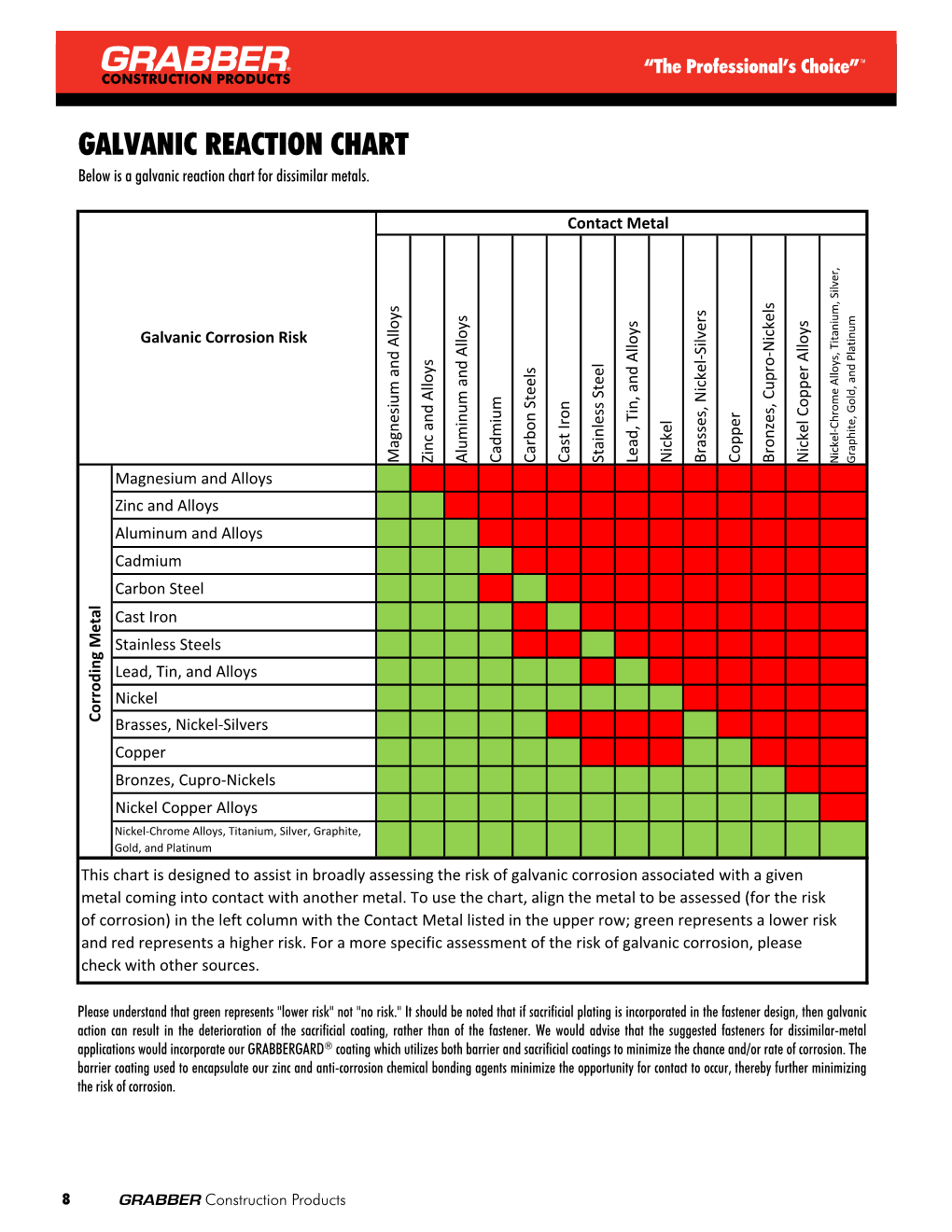
Galvanic Chart
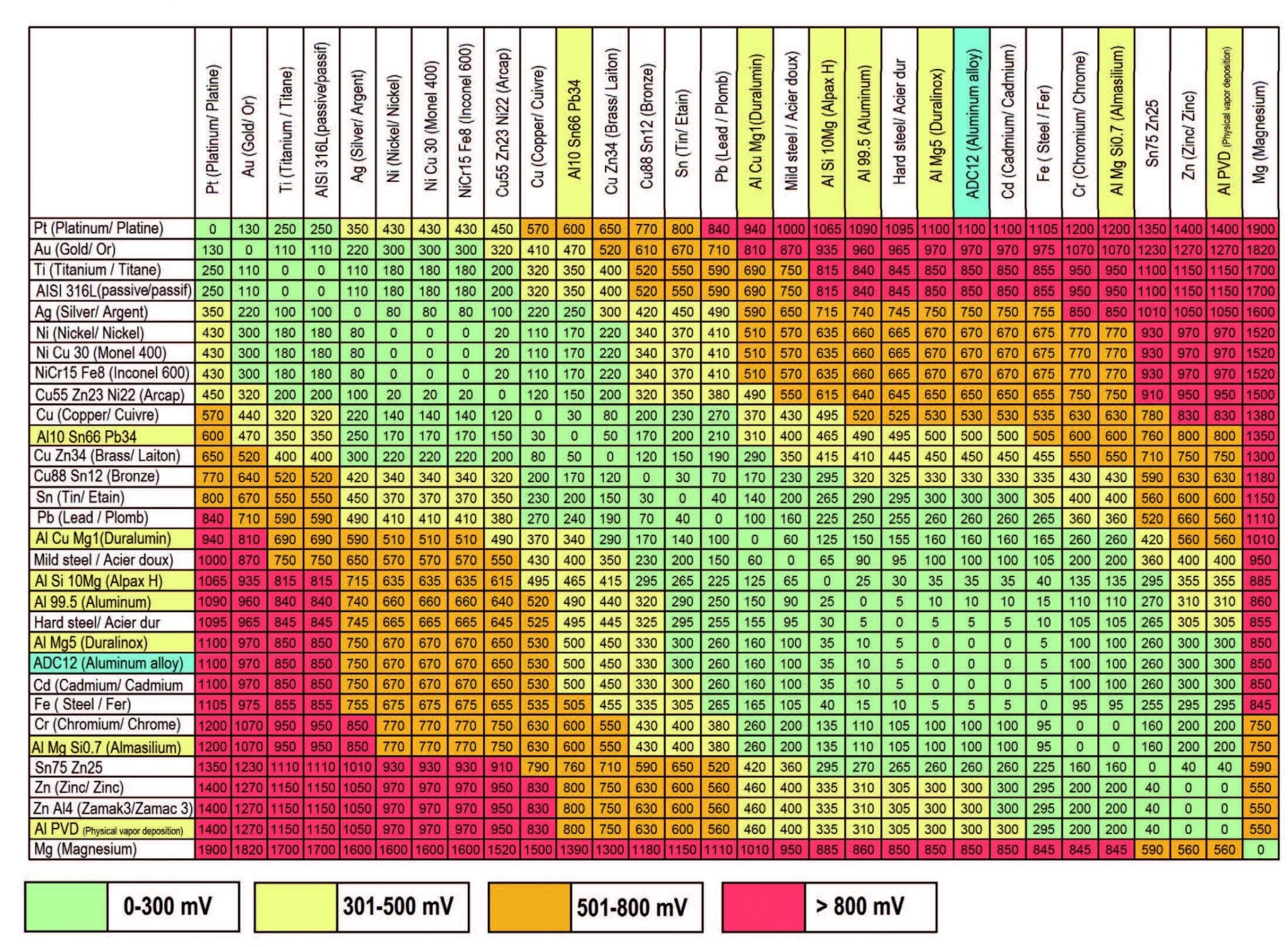
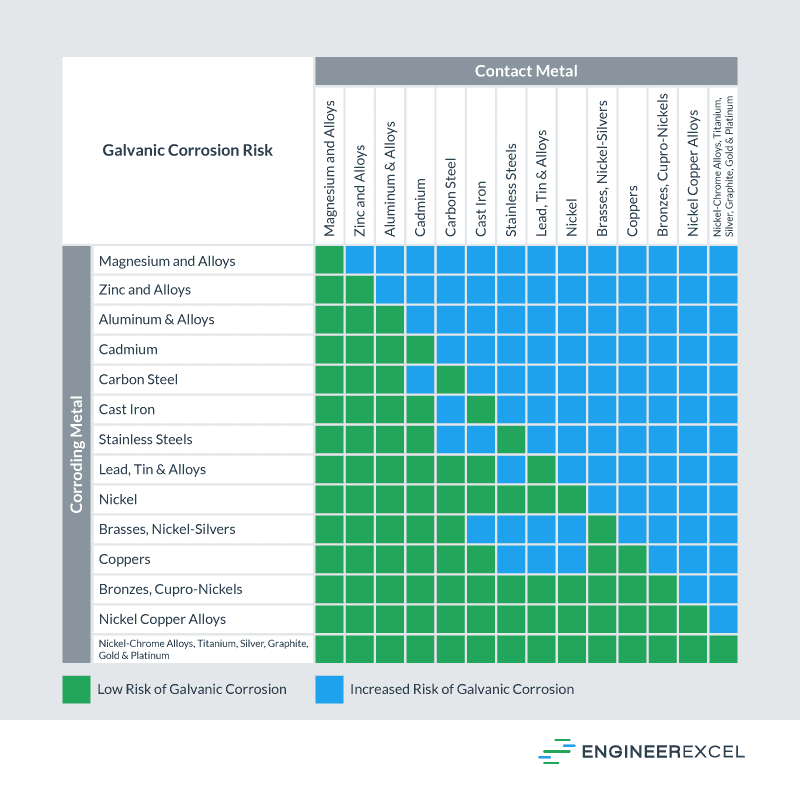
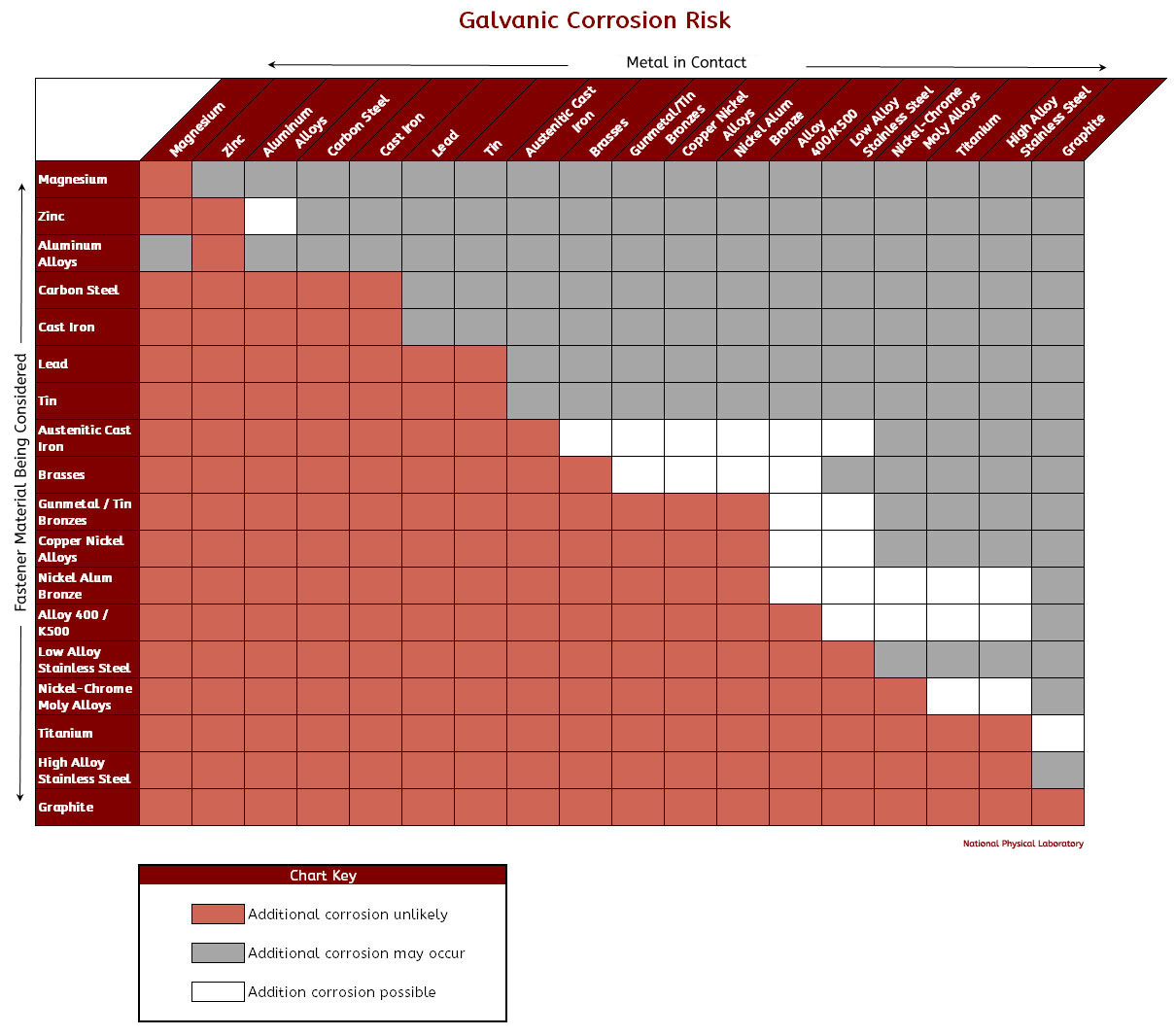
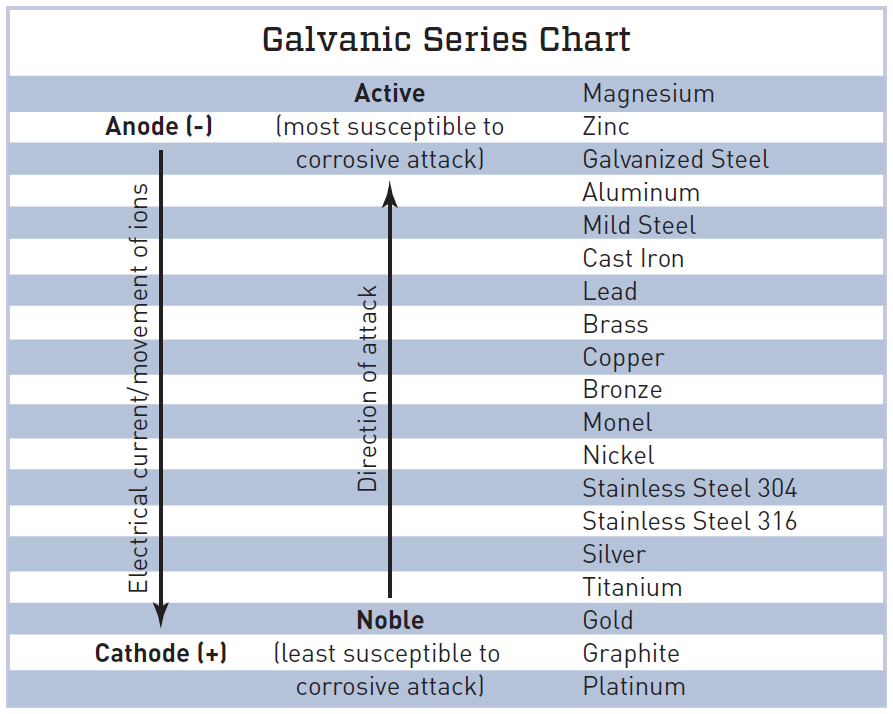
Galvanic Chart - When design requires that dissimilar metals come in contact, galvanic compatibility can be managed by finishes and plating which protects the base materials from corrosion. When dissimilar metals are connected — either by simple contact or by wiring — and they are immersed in water, a current will flow whi. When two metals are submerged in an electrolyte, while also electrically connected by some. The following galvanic table lists metals in the order of their relative activity in seawater environment. These charts show which commonly used metals are compatible and which will result in galvanic corrosion when in contact. What exactly is the galvanic series? T the galvanic series chart. An anode, cathode, electrolyte, and return path. For galvanic corrosion to occur, four elements are necessary: The list begins with the more active (anodic) metal and proceeds down the to the. The following galvanic table lists metals in the order of their relative activity in seawater environment. For galvanic corrosion to occur, four elements are necessary: This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with. An anode, cathode, electrolyte, and return path. When two metals are submerged in an electrolyte, while also electrically connected by some. Below is a galvanic reaction chart for dissimilar metals. The galvanic corrosion process is a transfer of electrons between two electrodes. For any combination of dissimilar metals, the metal with the lower number will act as an anode. What exactly is the galvanic series? A typical rule of thumb is that voltage differences. These charts show which commonly used metals are compatible and which will result in galvanic corrosion when in contact. When design requires that dissimilar metals come in contact, galvanic compatibility can be managed by finishes and plating which protects the base materials from corrosion. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with. The list begins with the more active (anodic) metal and proceeds down the to the. (noble metals are those that are. T the galvanic series chart. For any combination of dissimilar metals, the metal with the lower number will act as an anode. The following galvanic table lists metals in the order of their relative activity in seawater environment. T the galvanic series chart. For any combination of dissimilar metals, the metal with the lower number will act as an anode. The galvanic corrosion process is a transfer of electrons between two electrodes. When dissimilar metals are connected — either by simple contact or by wiring — and they are immersed in water, a current will flow whi. These. The following galvanic table lists metals in the order of their relative activity in seawater environment. (noble metals are those that are. The list begins with the more active (anodic) metal and proceeds down the to the. T the galvanic series chart. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with. A typical rule of thumb is that voltage differences. When two metals are submerged in an electrolyte, while also electrically connected by some. T the galvanic series chart. For galvanic corrosion to occur, four elements are necessary: These charts show which commonly used metals are compatible and which will result in galvanic corrosion when in contact. What exactly is the galvanic series? The galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). When dissimilar metals are connected — either by simple contact or by wiring — and they are. The galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). (noble metals are those that are. The list begins with the more active (anodic) metal and proceeds down the to the. For any combination of dissimilar metals, the metal with the lower number will act as an anode. The following galvanic table. For any combination of dissimilar metals, the metal with the lower number will act as an anode. What exactly is the galvanic series? (noble metals are those that are. The list begins with the more active (anodic) metal and proceeds down the to the. The following galvanic table lists metals in the order of their relative activity in seawater environment. These charts show which commonly used metals are compatible and which will result in galvanic corrosion when in contact. (noble metals are those that are. When dissimilar metals are connected — either by simple contact or by wiring — and they are immersed in water, a current will flow whi. When design requires that dissimilar metals come in contact, galvanic. The galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). When dissimilar metals are connected — either by simple contact or by wiring — and they are immersed in water, a current will flow whi. A typical rule of thumb is that voltage differences. These charts show which commonly used metals are. The following galvanic table lists metals in the order of their relative activity in seawater environment. The list begins with the more active (anodic) metal and proceeds down the to the. When design requires that dissimilar metals come in contact, galvanic compatibility can be managed by finishes and plating which protects the base materials from corrosion. An anode, cathode, electrolyte, and return path. A typical rule of thumb is that voltage differences. T the galvanic series chart. For any combination of dissimilar metals, the metal with the lower number will act as an anode. Below is a galvanic reaction chart for dissimilar metals. The galvanic corrosion process is a transfer of electrons between two electrodes. (noble metals are those that are. For galvanic corrosion to occur, four elements are necessary: When two metals are submerged in an electrolyte, while also electrically connected by some. These charts show which commonly used metals are compatible and which will result in galvanic corrosion when in contact.GALVANIC REACTION CHART Below Is a Galvanic Reaction Chart for Dissimilar Metals DocsLib
Stainless Steel Galvanic Corrosion Chart
Galvanic Series, or Nobility Chart for Dissimilar Metals Fair Wind Fasteners
Galvanic Corrosion Chart Dissimilar Metals A Visual Reference of Charts Chart Master
Galvanic Action Corrosion Prevention Architect's Blog
Galvanic Chart FINE METAL ROOF TECH
galvanic scale chart Separating galvanic metals
Galvanic Corrosion Chart
Separating Galvanic Metals JLC Online
how to prevent galvanic corrosion between aluminum and steel
This Chart Is Designed To Assist In Broadly Assessing The Risk Of Galvanic Corrosion Associated With A Given Metal Coming Into Contact With.
What Exactly Is The Galvanic Series?
The Galvanic Series Chart Below Shows Metals And Their Electrochemical Voltage Range (Relative Activity In Flowing Sea Water).
When Dissimilar Metals Are Connected — Either By Simple Contact Or By Wiring — And They Are Immersed In Water, A Current Will Flow Whi.
Related Post: