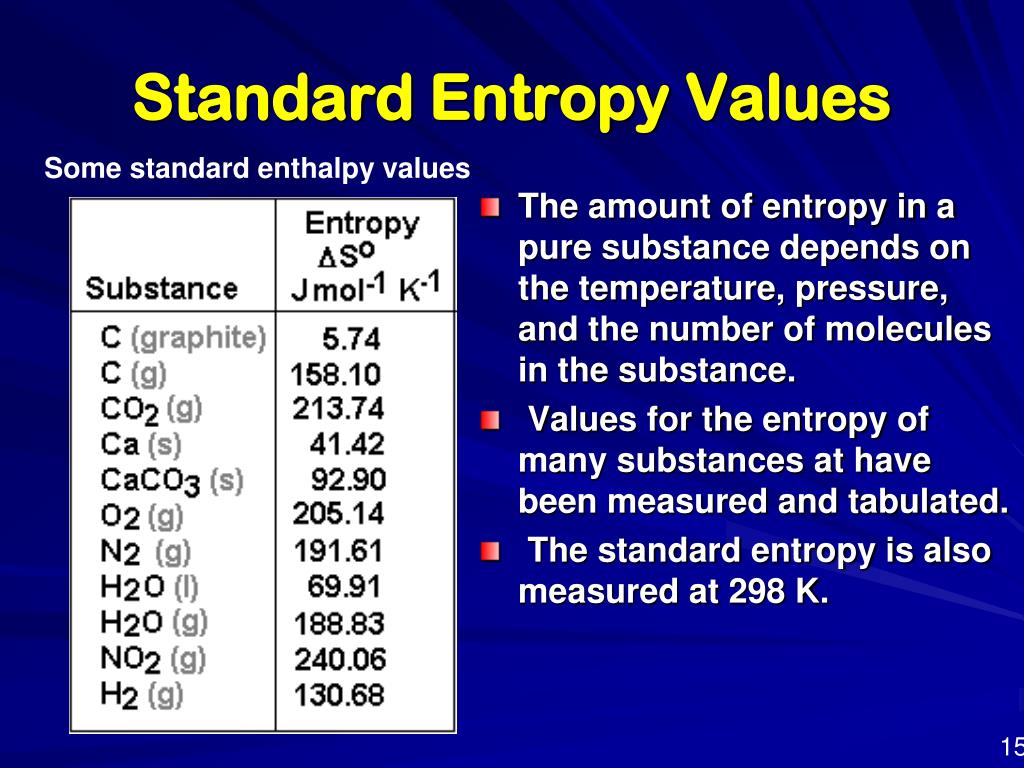
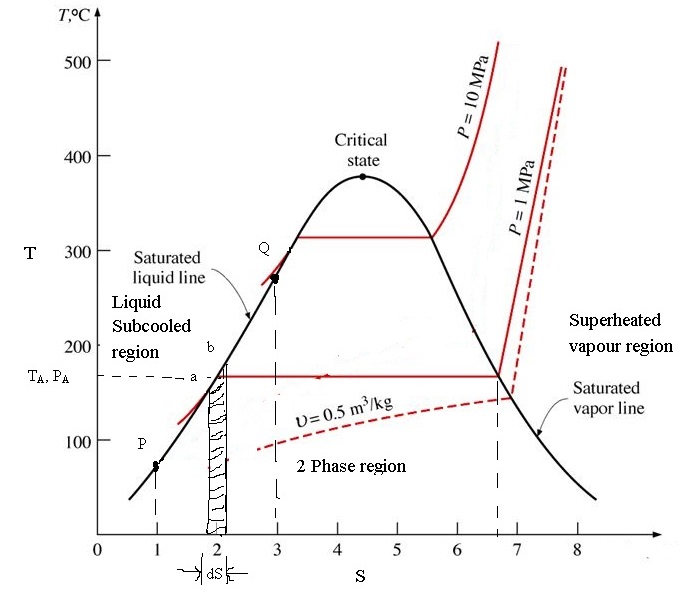
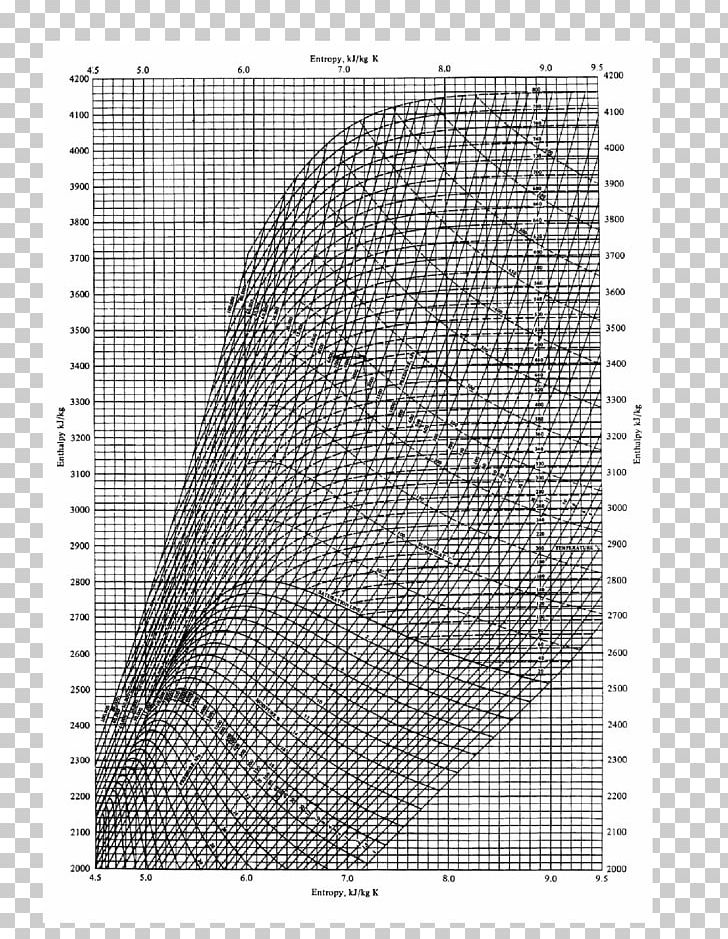
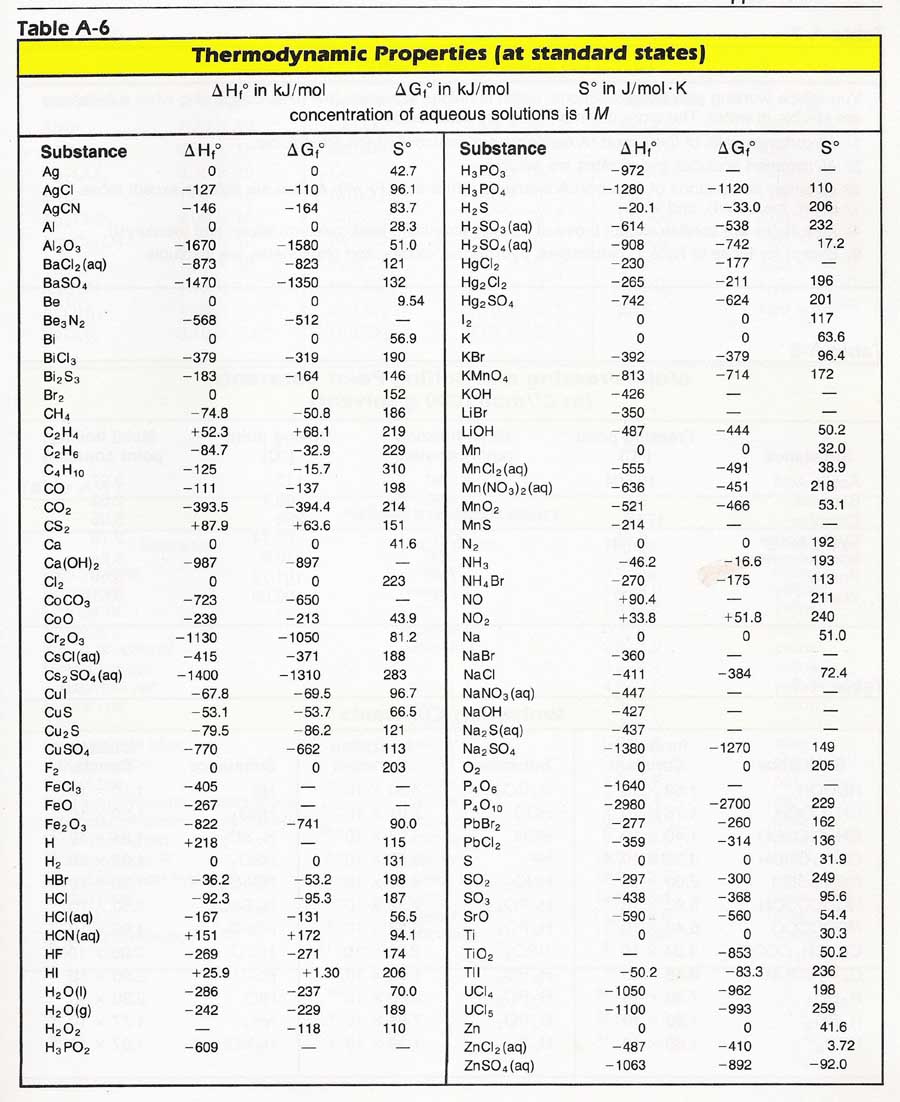
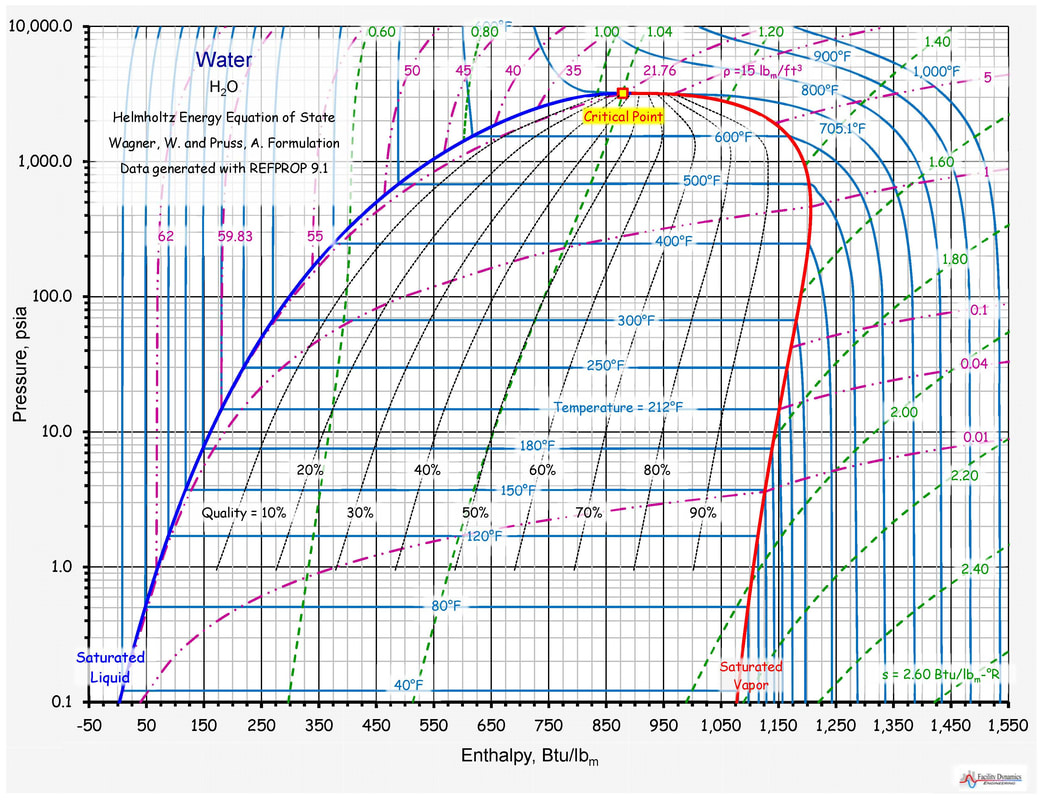
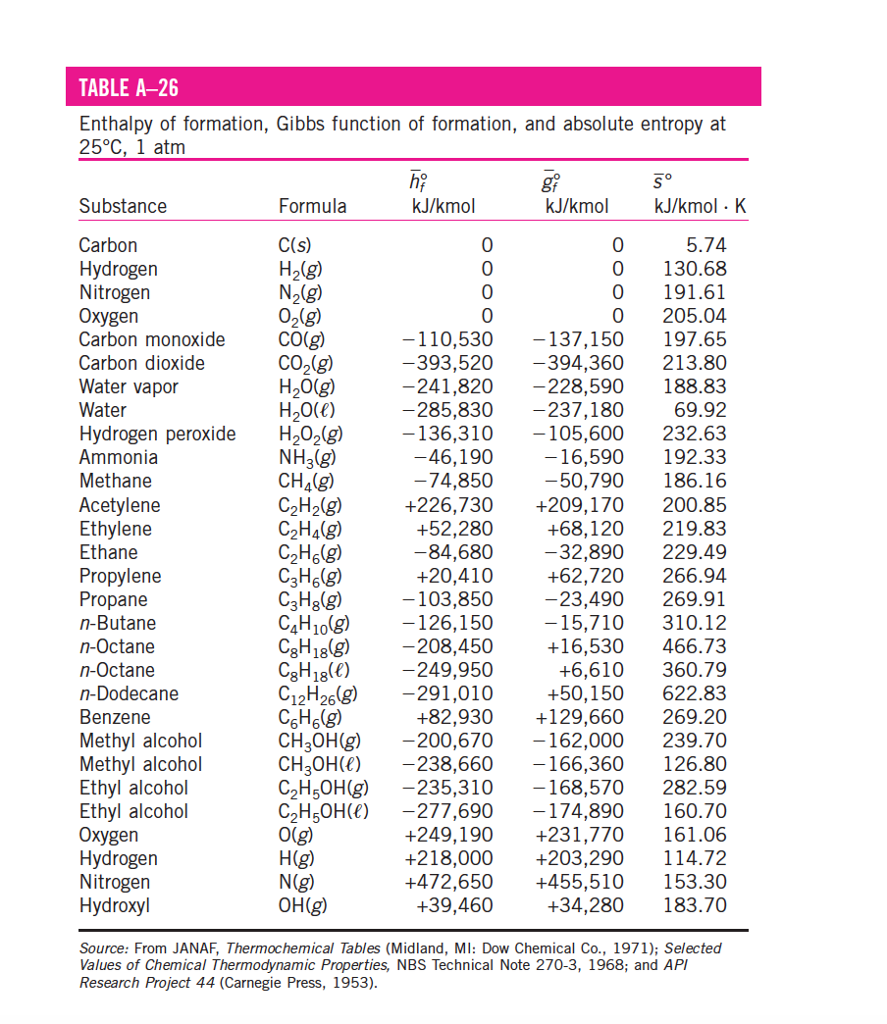
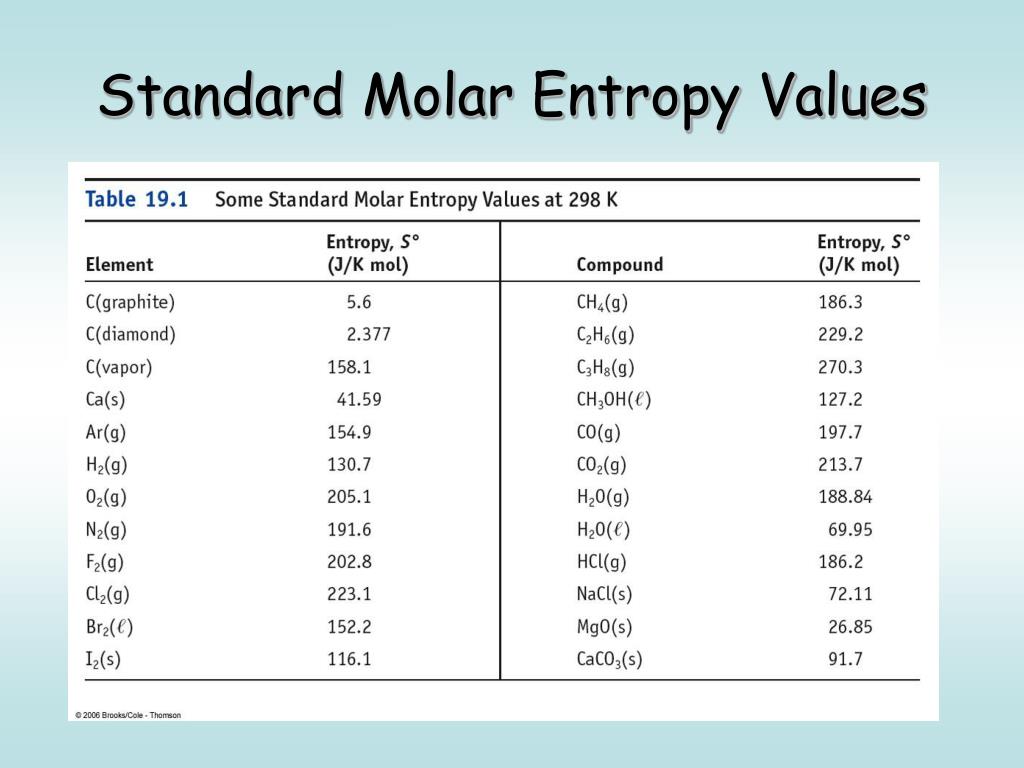
Entropy Charts
Entropy Charts - Entropy, the measure of a system’s thermal energy per unit temperature that is unavailable for doing useful work. Entropy is the disorder of a system, but that means a lot more than making a mess of a room. The meaning of entropy is a measure of the unavailable energy in a closed thermodynamic system that is also usually considered to be a measure of the system's. It is a measure of the unavailable energy in a closed thermodynamic system that. It is an extensive property of a thermodynamic system, meaning its value changes depending on the amount of matter. Entropy is the measure of the disorder of a system. Entropy is a key concept in physics and chemistry, with application in other. Entropy is defined as a measure of a system’s disorder or the energy unavailable to do work. It is a concept that permeates every facet of reality, shaping the flow of time, the behavior of systems, and even. The term and the concept are used in diverse fields, from classical. Entropy is a key concept in physics and chemistry, with application in other. The second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous process; Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. Entropy is a scientific concept commonly associated with disorder, randomness, or uncertainty. The second law of thermodynamics is best expressed in terms of a change in the thermodynamic variable known as entropy, which is represented by the symbol s. Entropy is not just an abstract principle tucked away in physics textbooks. Entropy is the disorder of a system, but that means a lot more than making a mess of a room. It is a concept that permeates every facet of reality, shaping the flow of time, the behavior of systems, and even. The term and the concept are used in diverse fields, from classical. It is a measure of the unavailable energy in a closed thermodynamic system that. It is a concept that permeates every facet of reality, shaping the flow of time, the behavior of systems, and even. Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. It is a measure of the unavailable energy in a closed thermodynamic system that. The term and the concept are used in diverse fields,. It is a concept that permeates every facet of reality, shaping the flow of time, the behavior of systems, and even. Entropy is defined as a measure of a system’s disorder or the energy unavailable to do work. The second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous process;. It is a measure of the unavailable energy in a closed thermodynamic system that. Entropy is the disorder of a system, but that means a lot more than making a mess of a room. It is an extensive property of a thermodynamic system, meaning its value changes depending on the amount of matter. Entropy is a key concept in physics. The second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous process; Entropy is not just an abstract principle tucked away in physics textbooks. Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The second law of thermodynamics is best expressed in terms of. Because work is obtained from ordered molecular motion,. Entropy is the disorder of a system, but that means a lot more than making a mess of a room. Entropy, the measure of a system’s thermal energy per unit temperature that is unavailable for doing useful work. Entropy is the measure of the disorder of a system. It is a concept. The second law of thermodynamics is best expressed in terms of a change in the thermodynamic variable known as entropy, which is represented by the symbol s. The meaning of entropy is a measure of the unavailable energy in a closed thermodynamic system that is also usually considered to be a measure of the system's. Entropy is defined as a. Entropy is the disorder of a system, but that means a lot more than making a mess of a room. The second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous process; It is a measure of the unavailable energy in a closed thermodynamic system that. Entropy is a scientific. The term and the concept are used in diverse fields, from classical. The meaning of entropy is a measure of the unavailable energy in a closed thermodynamic system that is also usually considered to be a measure of the system's. Entropy is defined as a measure of a system’s disorder or the energy unavailable to do work. Entropy is a. Entropy is defined as a measure of a system’s disorder or the energy unavailable to do work. Entropy is a key concept in physics and chemistry, with application in other. Entropy is the measure of the disorder of a system. The second law of thermodynamics is best expressed in terms of a change in the thermodynamic variable known as entropy,. Entropy is defined as a measure of a system’s disorder or the energy unavailable to do work. It is an extensive property of a thermodynamic system, meaning its value changes depending on the amount of matter. The second law of thermodynamics is best expressed in terms of a change in the thermodynamic variable known as entropy, which is represented by. It is an extensive property of a thermodynamic system, meaning its value changes depending on the amount of matter. Entropy, the measure of a system’s thermal energy per unit temperature that is unavailable for doing useful work. The meaning of entropy is a measure of the unavailable energy in a closed thermodynamic system that is also usually considered to be a measure of the system's. Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical. It is a concept that permeates every facet of reality, shaping the flow of time, the behavior of systems, and even. Entropy is a scientific concept commonly associated with disorder, randomness, or uncertainty. Entropy is defined as a measure of a system’s disorder or the energy unavailable to do work. Entropy is the disorder of a system, but that means a lot more than making a mess of a room. The second law of thermodynamics is best expressed in terms of a change in the thermodynamic variable known as entropy, which is represented by the symbol s. Entropy is not just an abstract principle tucked away in physics textbooks. It is a measure of the unavailable energy in a closed thermodynamic system that.Standard Entropy Chart
Explain Water Temperature Enthalpy Diagram For Water Methane
PPT Entropy PowerPoint Presentation, free download ID2017581
Temperature Entropy Diagram
Enthalpyentropy Chart Diagram Thermodynamics Water PNG, Clipart, Angle, Area, Artwork
Entropy Table
Enthalpy Entropy Diagram For Water And Steam Temperature Ent
Standard Entropy Chart
Enthalpy And Entropy Chart
Standard entropy table Lasialabama
The Second Law Of Thermodynamics States That The Total Entropy Of A System Either Increases Or Remains Constant In Any Spontaneous Process;
Entropy Is A Key Concept In Physics And Chemistry, With Application In Other.
Entropy Is The Measure Of The Disorder Of A System.
Because Work Is Obtained From Ordered Molecular Motion,.
Related Post: