Cdrh Organization Chart
Cdrh Organization Chart - Food and drug administration (fda) and ensures the safety and efficacy of medical devices and radiation. The center for devices and radiological health (cdrh) is a branch of the u.s. This catalog collates a variety of regulatory science tools that the fda's center for devices and radiological health's (cdrh) office of science and engineering labs (osel) developed and. Food and drug administration (fda), an agency that is part of the u.s. The cdrh is an organizational component of the fda that has been given the legal authority to regulate these products under the united states federal, food, drug and cosmetic (fd&c). Fda’s center for devices and radiological health (cdrh) regulates medical devices to assure their safety and effectiveness. Food and drug administration (fda) responsible for overseeing the regulation of medical devices and. The center for devices and radiological health (cdrh) works under the u.s. Importers of laser products will need to prepare and submit a 2877 form to us customs and/or the local fda office. The center for devices and radiological health (cdrh) is one of six product centers of the u.s. Food and drug administration (fda) responsible for overseeing the regulation of medical devices and. Importers of laser products will need to prepare and submit a 2877 form to us customs and/or the local fda office. The center for devices and radiological health (cdrh) is one of six product centers of the u.s. Food and drug administration (fda), an agency that is part of the u.s. This catalog collates a variety of regulatory science tools that the fda's center for devices and radiological health's (cdrh) office of science and engineering labs (osel) developed and. Fda’s center for devices and radiological health (cdrh) regulates medical devices to assure their safety and effectiveness. The cdrh is an organizational component of the fda that has been given the legal authority to regulate these products under the united states federal, food, drug and cosmetic (fd&c). A listing of databases for such topics as advisory committees, regulations, good practices, medical devices, premarket approval (pma) and notification (510 (k)), product. In keeping with our mission, the center for devices and radiological health (cdrh) is responsible for protecting and promoting the public health by assuring that patients. Food and drug administration (fda) and ensures the safety and efficacy of medical devices and radiation. The center for devices and radiological health (cdrh) works under the u.s. In keeping with our mission, the center for devices and radiological health (cdrh) is responsible for protecting and promoting the public health by assuring that patients. Fda’s center for devices and radiological health (cdrh) regulates medical devices to assure their safety and effectiveness. A listing of databases for. The center for devices and radiological health (cdrh) works under the u.s. The center for devices and radiological health (cdrh) is a branch of the u.s. Importers of laser products will need to prepare and submit a 2877 form to us customs and/or the local fda office. This catalog collates a variety of regulatory science tools that the fda's center. The center for devices and radiological health (cdrh) is one of six product centers of the u.s. This catalog collates a variety of regulatory science tools that the fda's center for devices and radiological health's (cdrh) office of science and engineering labs (osel) developed and. Food and drug administration (fda) responsible for overseeing the regulation of medical devices and. The. Importers of laser products will need to prepare and submit a 2877 form to us customs and/or the local fda office. We offer compliance services for customers who seek guidance in the certification of their new or existing laser system with the center for devices and radiological health (cdrh). Food and drug administration (fda) responsible for overseeing the regulation of. The center for devices and radiological health (cdrh) is one of six product centers of the u.s. This catalog collates a variety of regulatory science tools that the fda's center for devices and radiological health's (cdrh) office of science and engineering labs (osel) developed and. We offer compliance services for customers who seek guidance in the certification of their new. This catalog collates a variety of regulatory science tools that the fda's center for devices and radiological health's (cdrh) office of science and engineering labs (osel) developed and. A listing of databases for such topics as advisory committees, regulations, good practices, medical devices, premarket approval (pma) and notification (510 (k)), product. We offer compliance services for customers who seek guidance. The center for devices and radiological health (cdrh) is a branch of the u.s. The cdrh is an organizational component of the fda that has been given the legal authority to regulate these products under the united states federal, food, drug and cosmetic (fd&c). The center for devices and radiological health (cdrh) is one of six product centers of the. A listing of databases for such topics as advisory committees, regulations, good practices, medical devices, premarket approval (pma) and notification (510 (k)), product. Fda’s center for devices and radiological health (cdrh) regulates medical devices to assure their safety and effectiveness. The center for devices and radiological health (cdrh) works under the u.s. Importers of laser products will need to prepare. The center for devices and radiological health (cdrh) is a branch of the u.s. We offer compliance services for customers who seek guidance in the certification of their new or existing laser system with the center for devices and radiological health (cdrh). A listing of databases for such topics as advisory committees, regulations, good practices, medical devices, premarket approval (pma). The center for devices and radiological health (cdrh) works under the u.s. Importers of laser products will need to prepare and submit a 2877 form to us customs and/or the local fda office. The center for devices and radiological health (cdrh) is one of six product centers of the u.s. Food and drug administration (fda) and ensures the safety and. We offer compliance services for customers who seek guidance in the certification of their new or existing laser system with the center for devices and radiological health (cdrh). In keeping with our mission, the center for devices and radiological health (cdrh) is responsible for protecting and promoting the public health by assuring that patients. The center for devices and radiological health (cdrh) is a branch of the u.s. The cdrh is an organizational component of the fda that has been given the legal authority to regulate these products under the united states federal, food, drug and cosmetic (fd&c). Food and drug administration (fda), an agency that is part of the u.s. Fda’s center for devices and radiological health (cdrh) regulates medical devices to assure their safety and effectiveness. This catalog collates a variety of regulatory science tools that the fda's center for devices and radiological health's (cdrh) office of science and engineering labs (osel) developed and. Food and drug administration (fda) responsible for overseeing the regulation of medical devices and. The center for devices and radiological health (cdrh) works under the u.s. Food and drug administration (fda) and ensures the safety and efficacy of medical devices and radiation.FDA CDRH Organizational Structure & Overview
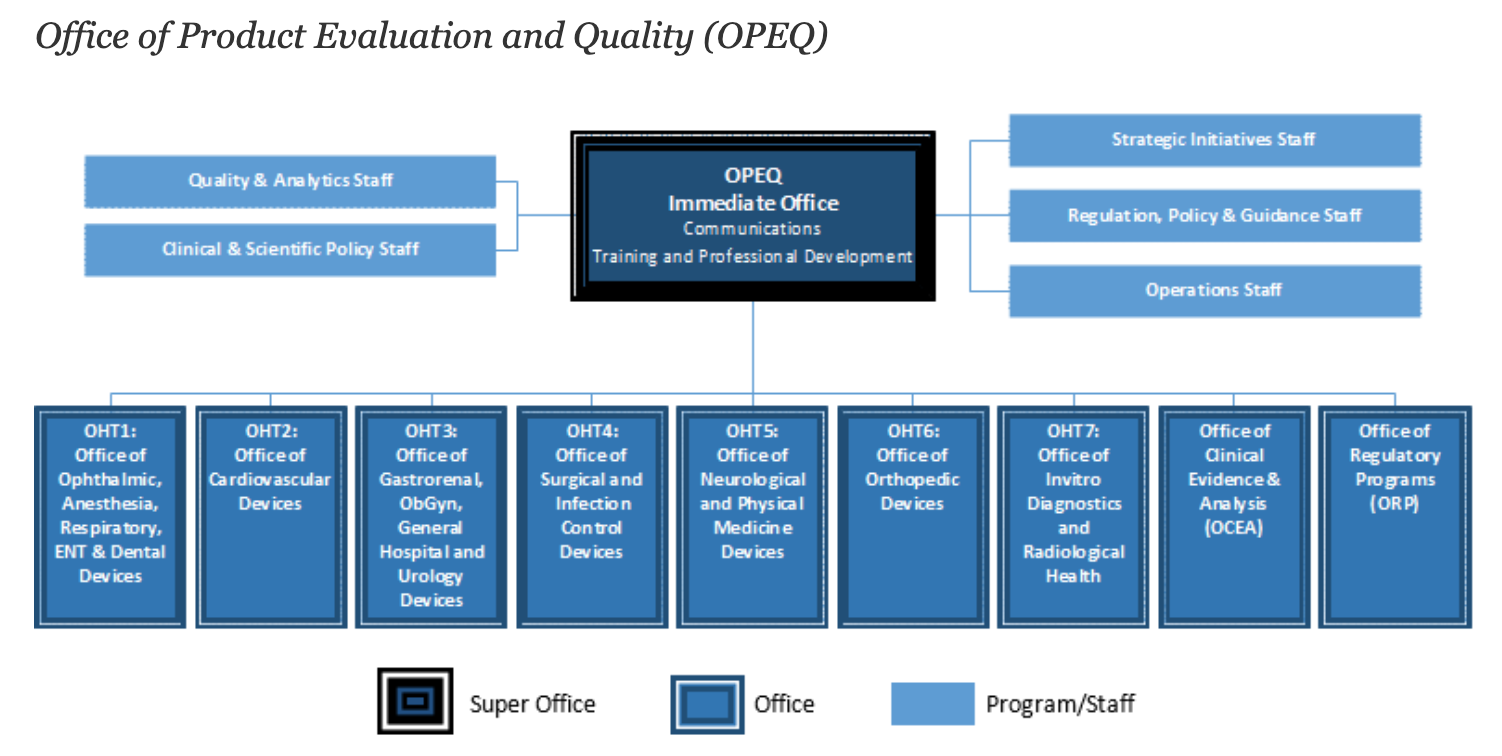
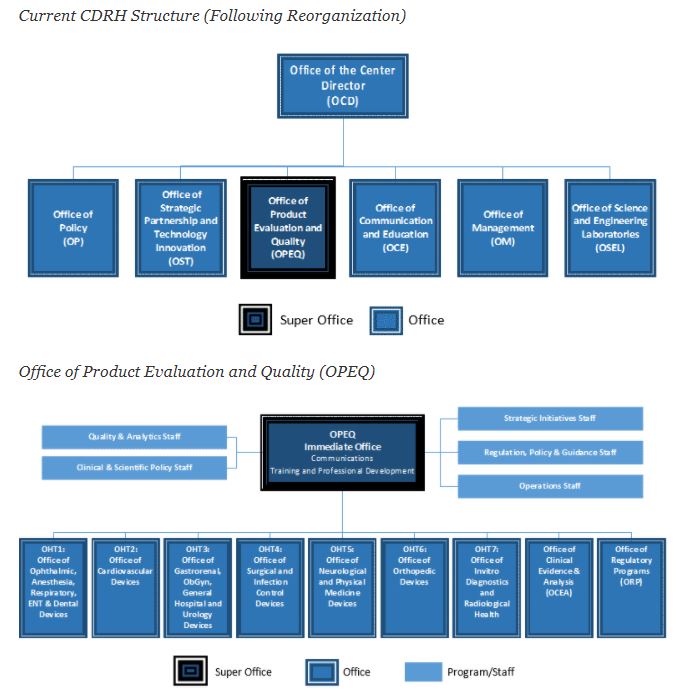
8 Important Facts About FDA’s New Office of Product Evaluation and Quality
PPT Regulatory Issues PowerPoint Presentation, free download ID1606212
PreApplication Information Webinar for PAR21183, "Developing Digital Therapeutics for
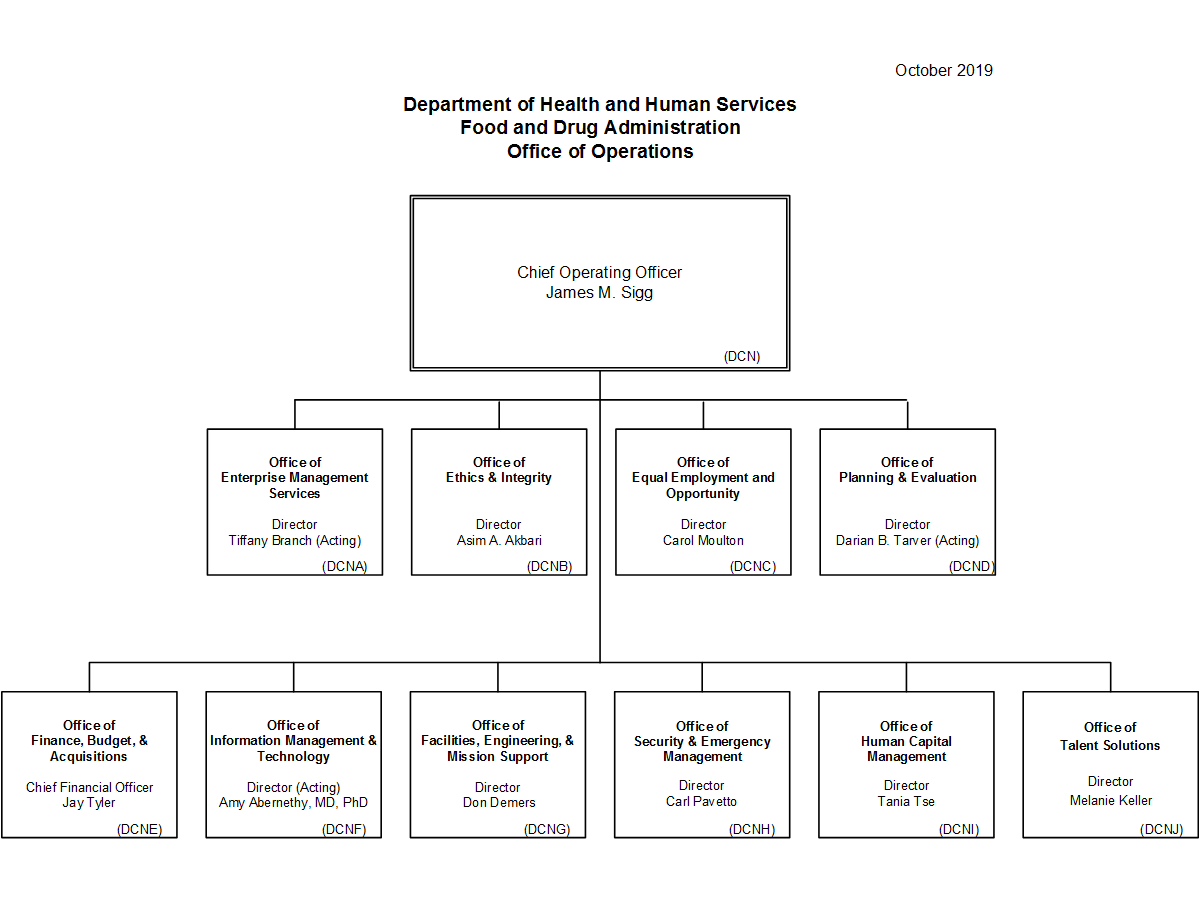
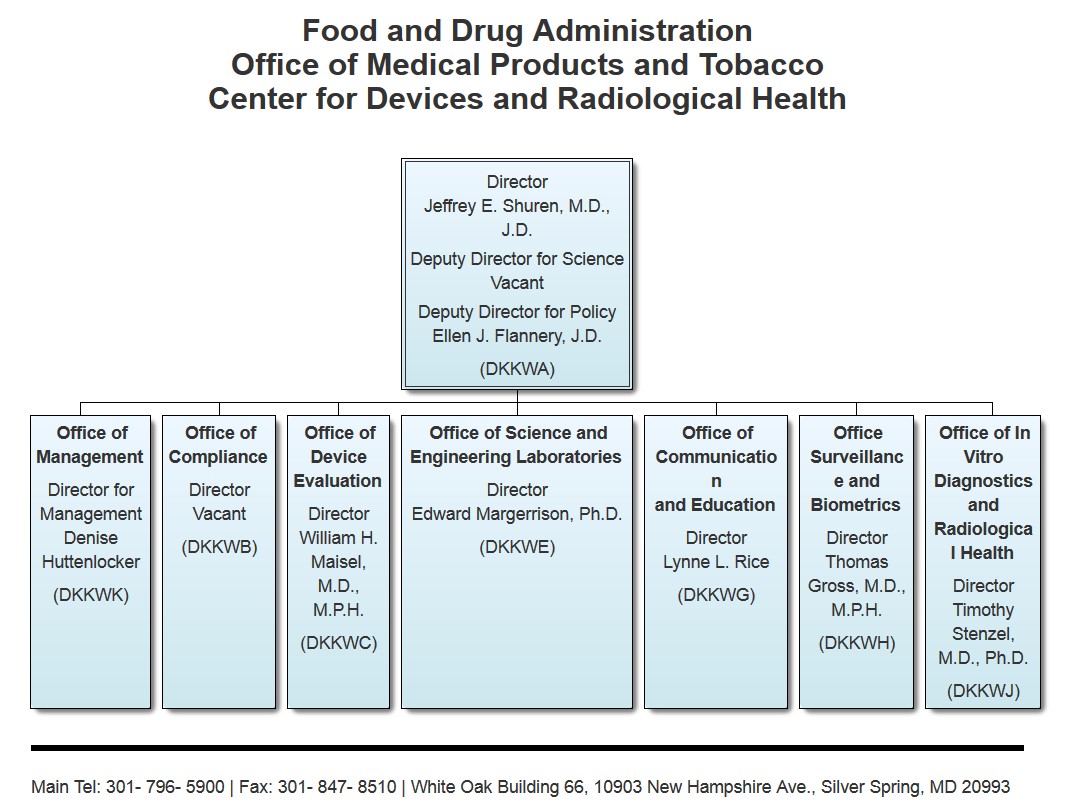
Office of Operations Organization Chart FDA
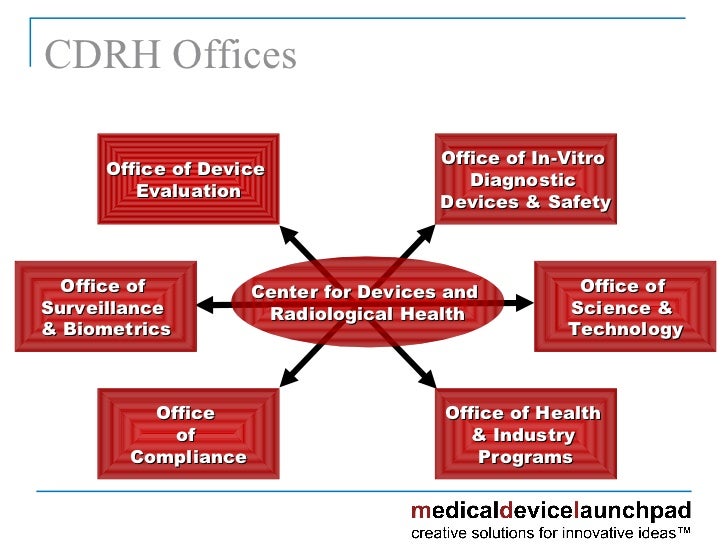
Center For Device and Radiological Health Dawnbreaker MRR
Introduction to US FDA Regulatory Framework ppt download
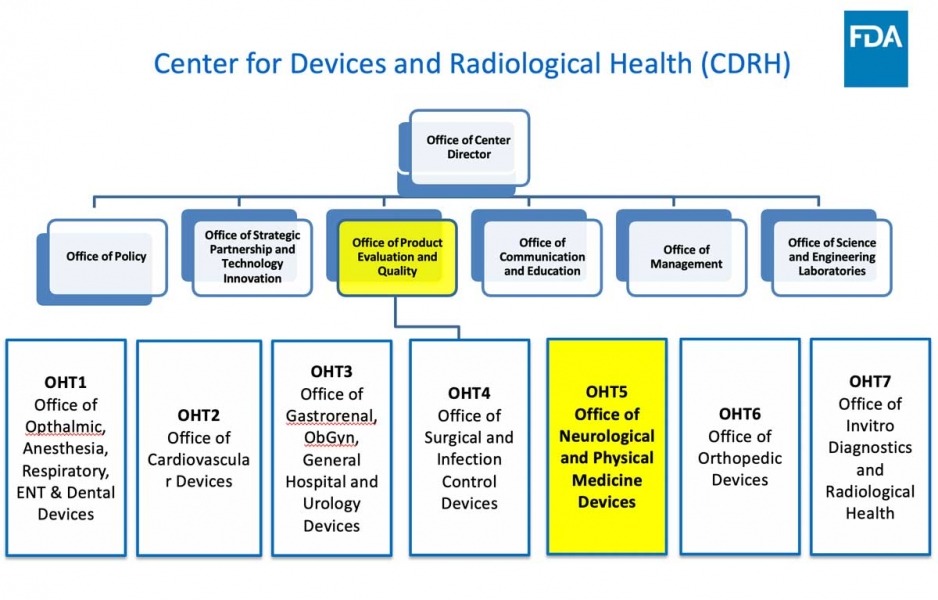
It’s Now Official The New CDRH Organizational Structure and How It May Impact Your Interactions
Center For Device and Radiological Health Dawnbreaker MRR
Understanding FDA Requirements Medical Devices
A Listing Of Databases For Such Topics As Advisory Committees, Regulations, Good Practices, Medical Devices, Premarket Approval (Pma) And Notification (510 (K)), Product.
Importers Of Laser Products Will Need To Prepare And Submit A 2877 Form To Us Customs And/Or The Local Fda Office.
The Center For Devices And Radiological Health (Cdrh) Is One Of Six Product Centers Of The U.s.
Related Post: