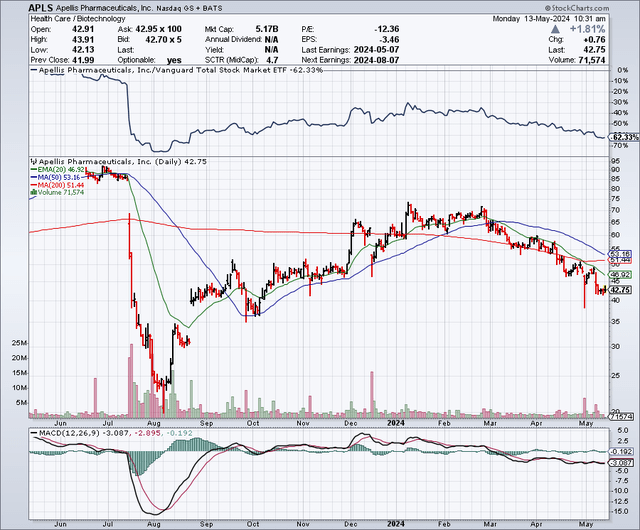
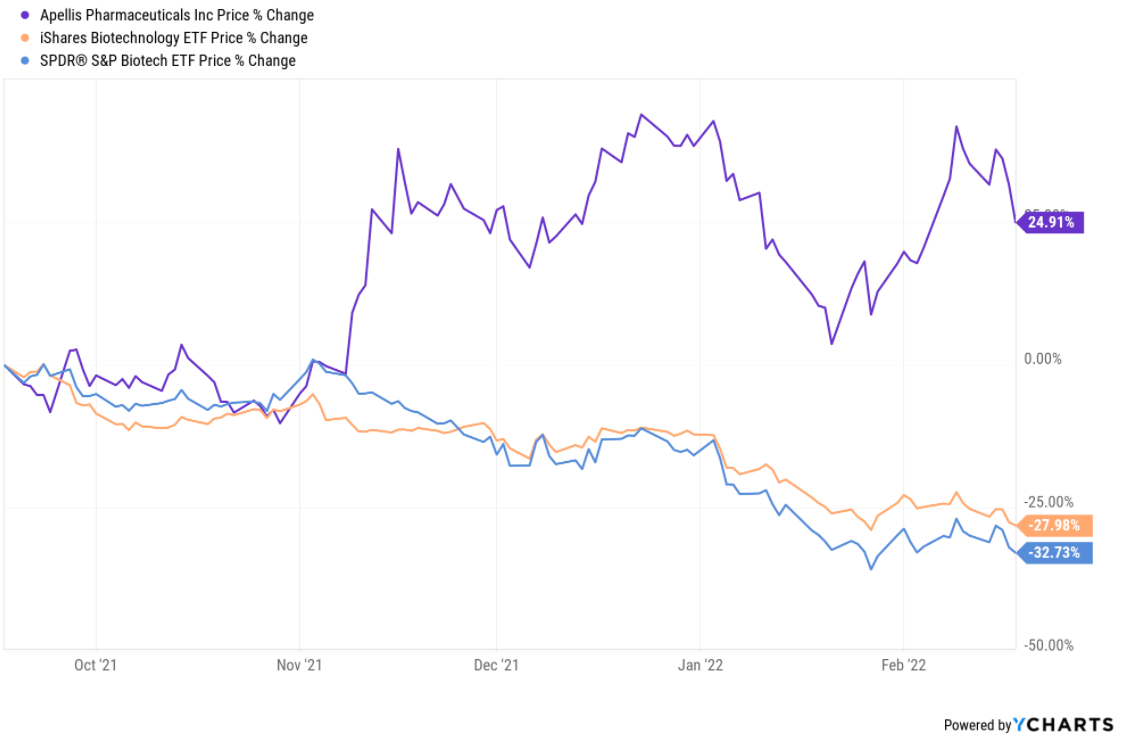
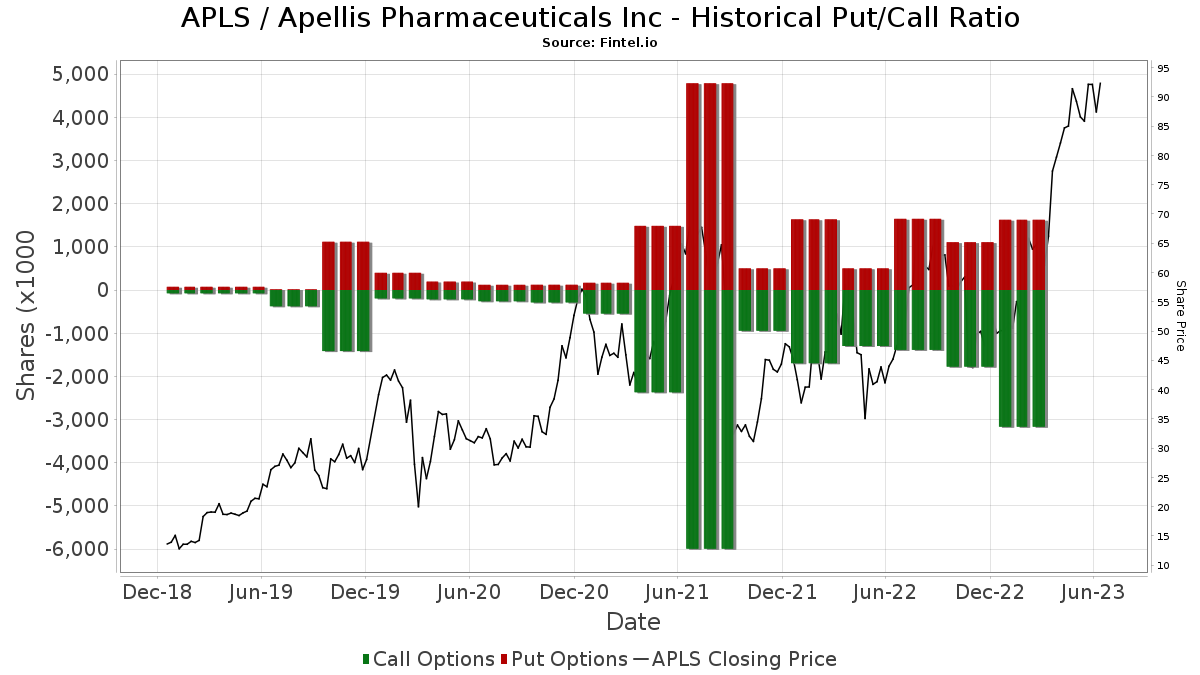
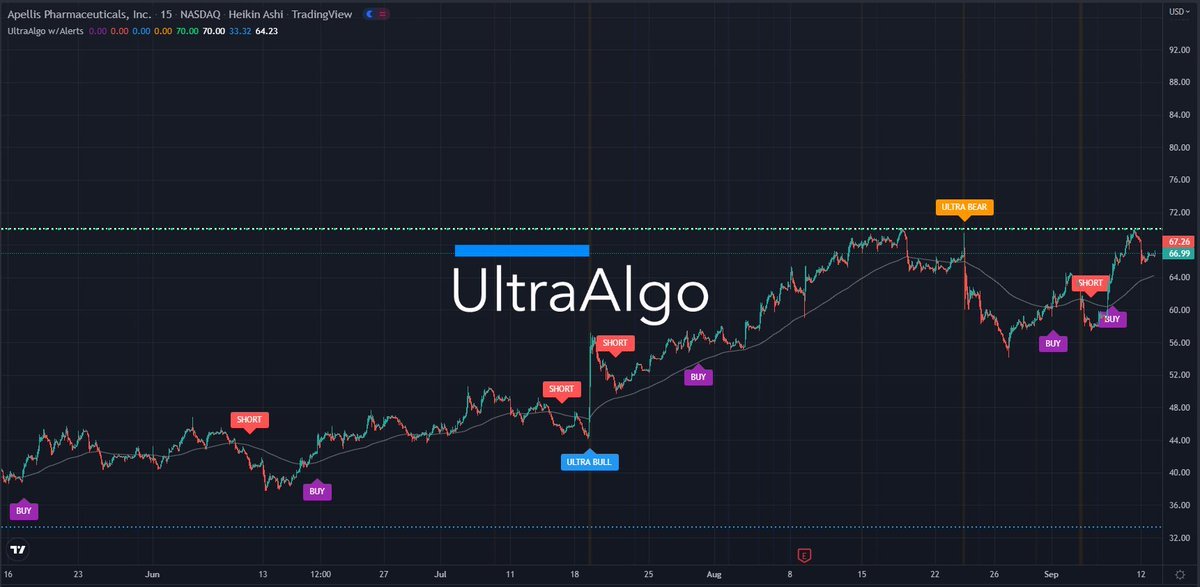
Apellis Stock Chart
Apellis Stock Chart - Detailed results from the pivotal phase 3 valiant study were presented at kidney week 2024,. Patients and healthcare providers in the united states can learn more at: Food and drug administration (fda) has. Please see full prescribing information. Commercialization rights for systemic pegcetacoplan and retains worldwide commercial rights for ophthalmological pegcetacoplan, including for. Portfolio insightsfree to usedynamic chartsstocks, etfs & options Syfovre.com comprehensive product support for patients empaveli apellis. By pioneering targeted c3 therapies, we aim to treat diseases that are driven by excessive. Apellis reported a net loss of $36.4 million and $197.9 million for the fourth quarter and full year 2024, respectively, compared to a net loss of $88.5 million and $528.6 million for. Apls) today announced that the u.s. By pioneering targeted c3 therapies, we aim to treat diseases that are driven by excessive. Syfovre.com comprehensive product support for patients empaveli apellis. Sales of aspaveli ranging from high teens to high twenties. Commercialization rights for systemic pegcetacoplan and retains worldwide commercial rights for ophthalmological pegcetacoplan, including for. Please see full prescribing information. Food and drug administration (fda) has. By pioneering targeted c3 therapies, we aim to treat diseases that are driven by excessive. Patients and healthcare providers in the united states can learn more at: Apellis reported a net loss of $36.4 million and $197.9 million for the fourth quarter and full year 2024, respectively, compared to a net loss of $88.5 million and $528.6 million for. Apls) today announced that the u.s. Patients and healthcare providers in the united states can learn more at: Apellis reported a net loss of $36.4 million and $197.9 million for the fourth quarter and full year 2024, respectively, compared to a net loss of $88.5 million and $528.6 million for. Syfovre.com comprehensive product support for patients empaveli apellis. Portfolio insightsfree to usedynamic chartsstocks, etfs & options. By pioneering targeted c3 therapies, we aim to treat diseases that are driven by excessive. Please see full prescribing information. Portfolio insightsfree to usedynamic chartsstocks, etfs & options We brought forward the first new class of complement medicine in 15 years with. At apellis, we aim to transform treatment across a broad range of debilitating diseases driven by complement. Commercialization rights for systemic pegcetacoplan and retains worldwide commercial rights for ophthalmological pegcetacoplan, including for. Portfolio insightsfree to usedynamic chartsstocks, etfs & options At apellis, we aim to transform treatment across a broad range of debilitating diseases driven by complement. Apellis reported a net loss of $36.4 million and $197.9 million for the fourth quarter and full year 2024, respectively,. Syfovre.com comprehensive product support for patients empaveli apellis. Food and drug administration (fda) has. Detailed results from the pivotal phase 3 valiant study were presented at kidney week 2024,. Apls) today announced that the u.s. At apellis, we aim to transform treatment across a broad range of debilitating diseases driven by complement. By pioneering targeted c3 therapies, we aim to treat diseases that are driven by excessive. Commercialization rights for systemic pegcetacoplan and retains worldwide commercial rights for ophthalmological pegcetacoplan, including for. Food and drug administration (fda) has. Detailed results from the pivotal phase 3 valiant study were presented at kidney week 2024,. Patients and healthcare providers in the united states can. Commercialization rights for systemic pegcetacoplan and retains worldwide commercial rights for ophthalmological pegcetacoplan, including for. Portfolio insightsfree to usedynamic chartsstocks, etfs & options Apellis reported a net loss of $36.4 million and $197.9 million for the fourth quarter and full year 2024, respectively, compared to a net loss of $88.5 million and $528.6 million for. Food and drug administration (fda). Please see full prescribing information. Apellis reported a net loss of $36.4 million and $197.9 million for the fourth quarter and full year 2024, respectively, compared to a net loss of $88.5 million and $528.6 million for. Detailed results from the pivotal phase 3 valiant study were presented at kidney week 2024,. At apellis, we aim to transform treatment across. Patients and healthcare providers in the united states can learn more at: By pioneering targeted c3 therapies, we aim to treat diseases that are driven by excessive. Food and drug administration (fda) has. Please see full prescribing information. Apls) today announced that the u.s. Apls) today announced that the u.s. Apellis reported a net loss of $36.4 million and $197.9 million for the fourth quarter and full year 2024, respectively, compared to a net loss of $88.5 million and $528.6 million for. At apellis, we aim to transform treatment across a broad range of debilitating diseases driven by complement. Sales of aspaveli ranging from. We brought forward the first new class of complement medicine in 15 years with. Syfovre.com comprehensive product support for patients empaveli apellis. Commercialization rights for systemic pegcetacoplan and retains worldwide commercial rights for ophthalmological pegcetacoplan, including for. Apellis reported a net loss of $36.4 million and $197.9 million for the fourth quarter and full year 2024, respectively, compared to a. Detailed results from the pivotal phase 3 valiant study were presented at kidney week 2024,. Please see full prescribing information. Commercialization rights for systemic pegcetacoplan and retains worldwide commercial rights for ophthalmological pegcetacoplan, including for. By pioneering targeted c3 therapies, we aim to treat diseases that are driven by excessive. Syfovre.com comprehensive product support for patients empaveli apellis. Food and drug administration (fda) has. At apellis, we aim to transform treatment across a broad range of debilitating diseases driven by complement. Apellis reported a net loss of $36.4 million and $197.9 million for the fourth quarter and full year 2024, respectively, compared to a net loss of $88.5 million and $528.6 million for. We brought forward the first new class of complement medicine in 15 years with. Patients and healthcare providers in the united states can learn more at: Portfolio insightsfree to usedynamic chartsstocks, etfs & options9 Analysts Have This to Say About Apellis Pharmaceuticals Markets Insider
Why (Ape)llis Pharmaceuticals APLS stock is about to jump r/wallstreetbets
Basics to Investing Apellis Pharmaceuticals, Inc. APLS Stock Charts 0256 YouTube
Apellis Pharmaceuticals Strives For Recovery Despite Syfovre Setbacks (NASDAQAPLS) Seeking Alpha
APLS Apellis Pharmaceuticals stock (Breakout) r/StockConsultant
Apellis Pharmaceuticals Stock The Situation Has Improved Recently (NASDAQAPLS) Seeking Alpha
APLS Institutional Ownership and Shareholders Apellis Pharmaceuticals Inc (NASDAQ) Stock
APELLIS PHARMACEUTICALS INC. quote Financial instrument overview NASDAQ Stocks
Trading Ideas for APLS (Apellis Pharmaceuticals Inc)
APLS Stock Price Apellis Pharmaceuticals Inc Stock Candlestick Chart StockScan
Sales Of Aspaveli Ranging From High Teens To High Twenties.
By Pioneering Targeted C3 Therapies, We Aim To Treat Diseases That Are Driven By Excessive.
Apls) Today Announced That The U.s.
Related Post: