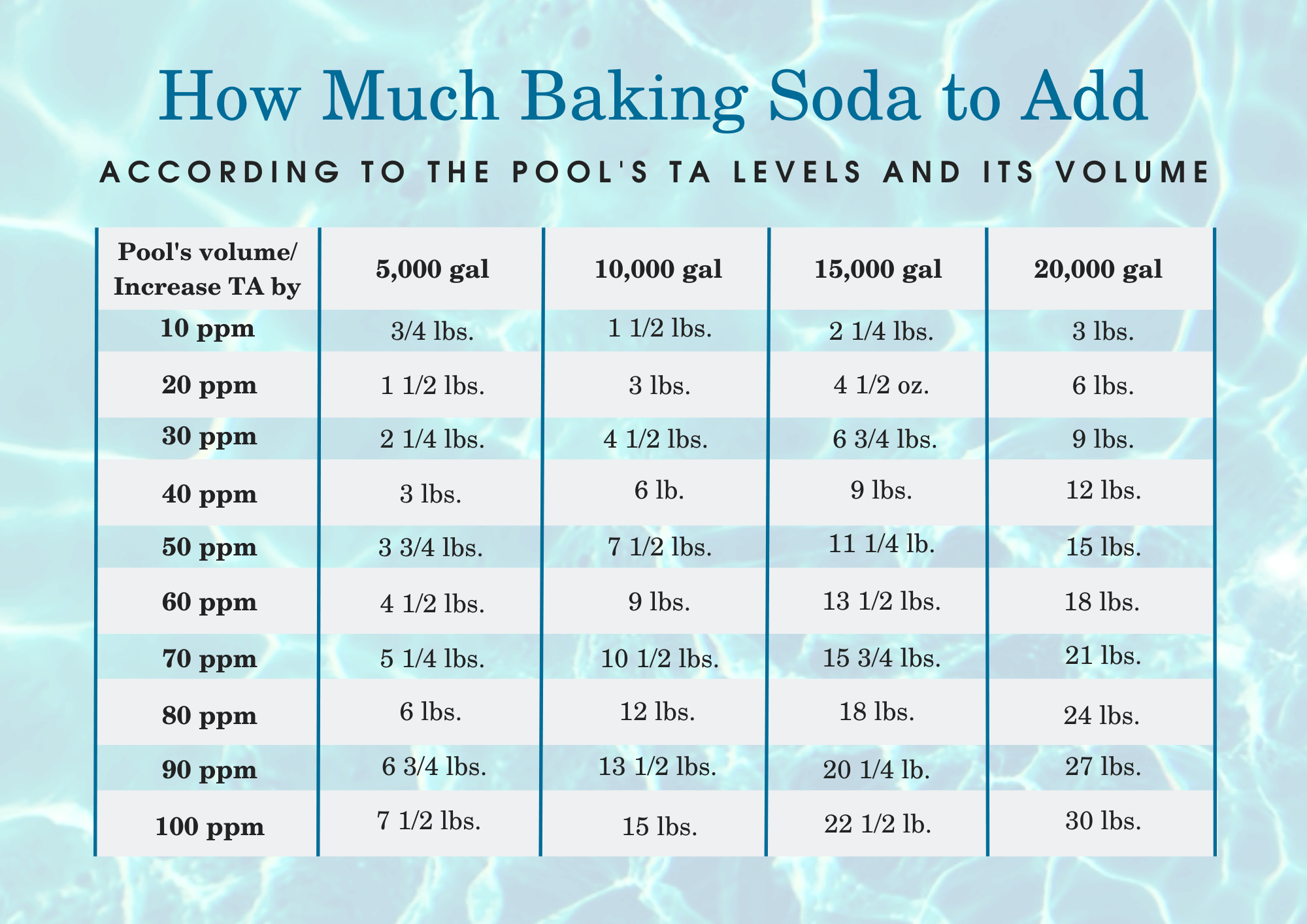Alkalinity Pool Chart
Alkalinity Pool Chart - Alkalinity is caused by strong bases and the salts of. In this article, we will explore the definition of alkalinity, its chemical. Alkalinity is the strength of a buffer solution composed of weak acids and their conjugate bases. It’s not a specific molecule but rather a measure of a water body’s ability to neutralize acids and. The alkalinity of water is a measure of how much acid it can neutralize. Total alkalinity is a measurement of the concentration of all alkaline substances dissolved in the water that can both attract and release hydrogen ions (h +). This is also called buffering capacity. Unlike ph, which measures acidity or alkalinity, alkalinity quantifies water's buffering capacity. Alkalinity refers to the quantitative measurement of the bicarbonate, carbonate, hydroxide, and hydrogen ions in a solution, expressed in equivalents per liter or milliequivalents per liter. It is measured by titrating the solution with an acid such as hcl until its ph changes abruptly, or. This ability is usually referred to as water’s “buffering. Unlike ph, which measures acidity or alkalinity, alkalinity quantifies water's buffering capacity. In this article, we will explore the definition of alkalinity, its chemical. Alkalinity is the strength of a buffer solution composed of weak acids and their conjugate bases. Alkalinity is caused by strong bases and the salts of. Alkalinity is a measure of water’s ability to resist ph changes that lead to acidity, or to neutralize acids, and maintain a fairly stable ph. The higher the alkalinity of a water body, the better it can protect itself against the. Alkalinity refers to the quantitative measurement of the bicarbonate, carbonate, hydroxide, and hydrogen ions in a solution, expressed in equivalents per liter or milliequivalents per liter. It is measured by titrating the solution with an acid such as hcl until its ph changes abruptly, or. Alkalinity measures water’s ability to maintain a stable ph level. Alkalinity is caused by strong bases and the salts of. If any changes are made to the water that could raise or lower the ph value, alkalinity acts as a buffer, protecting the. The higher the alkalinity of a water body, the better it can protect itself against the. It is measured by titrating the solution with an acid such. Alkalinity refers to the quantitative measurement of the bicarbonate, carbonate, hydroxide, and hydrogen ions in a solution, expressed in equivalents per liter or milliequivalents per liter. This is also called buffering capacity. Unlike ph, which measures acidity or alkalinity, alkalinity quantifies water's buffering capacity. In the world of water chemistry, alkalinity often plays a crucial, yet somewhat unnoticed, role. It. In this article, we will explore the definition of alkalinity, its chemical. It’s not a specific molecule but rather a measure of a water body’s ability to neutralize acids and. This is also called buffering capacity. Alkalinity is the strength of a buffer solution composed of weak acids and their conjugate bases. Alkalinity refers to the quantitative measurement of the. Alkalinity refers to the quantitative measurement of the bicarbonate, carbonate, hydroxide, and hydrogen ions in a solution, expressed in equivalents per liter or milliequivalents per liter. It’s not a specific molecule but rather a measure of a water body’s ability to neutralize acids and. The higher the alkalinity of a water body, the better it can protect itself against the.. The higher the alkalinity of a water body, the better it can protect itself against the. Alkalinity is caused by strong bases and the salts of. Alkalinity is produced by substances that yield hydroxyl ions or by definition, a substance is alkaline if it will neutralize hydrogen ions. In this article, we will explore the definition of alkalinity, its chemical.. Alkalinity refers to the quantitative measurement of the bicarbonate, carbonate, hydroxide, and hydrogen ions in a solution, expressed in equivalents per liter or milliequivalents per liter. Unlike ph, which measures acidity or alkalinity, alkalinity quantifies water's buffering capacity. Alkalinity is the strength of a buffer solution composed of weak acids and their conjugate bases. This ability is usually referred to. It’s not a specific molecule but rather a measure of a water body’s ability to neutralize acids and. Unlike ph, which measures acidity or alkalinity, alkalinity quantifies water's buffering capacity. Alkalinity refers to the quantitative measurement of the bicarbonate, carbonate, hydroxide, and hydrogen ions in a solution, expressed in equivalents per liter or milliequivalents per liter. The higher the alkalinity. Alkalinity is the strength of a buffer solution composed of weak acids and their conjugate bases. Alkalinity measures water’s ability to maintain a stable ph level. Total alkalinity is a measurement of the concentration of all alkaline substances dissolved in the water that can both attract and release hydrogen ions (h +). The alkalinity of water is a measure of. In the world of water chemistry, alkalinity often plays a crucial, yet somewhat unnoticed, role. This is also called buffering capacity. Alkalinity is caused by strong bases and the salts of. It is measured by titrating the solution with an acid such as hcl until its ph changes abruptly, or. Alkalinity is produced by substances that yield hydroxyl ions or. In the world of water chemistry, alkalinity often plays a crucial, yet somewhat unnoticed, role. Alkalinity is caused by strong bases and the salts of. Alkalinity refers to the quantitative measurement of the bicarbonate, carbonate, hydroxide, and hydrogen ions in a solution, expressed in equivalents per liter or milliequivalents per liter. Alkalinity is produced by substances that yield hydroxyl ions. Alkalinity is the strength of a buffer solution composed of weak acids and their conjugate bases. The alkalinity of water is a measure of how much acid it can neutralize. In this article, we will explore the definition of alkalinity, its chemical. Alkalinity refers to the quantitative measurement of the bicarbonate, carbonate, hydroxide, and hydrogen ions in a solution, expressed in equivalents per liter or milliequivalents per liter. This ability is usually referred to as water’s “buffering. Unlike ph, which measures acidity or alkalinity, alkalinity quantifies water's buffering capacity. It’s not a specific molecule but rather a measure of a water body’s ability to neutralize acids and. Total alkalinity is a measurement of the concentration of all alkaline substances dissolved in the water that can both attract and release hydrogen ions (h +). Alkalinity is a measure of water’s ability to resist ph changes that lead to acidity, or to neutralize acids, and maintain a fairly stable ph. The higher the alkalinity of a water body, the better it can protect itself against the. This is also called buffering capacity. If any changes are made to the water that could raise or lower the ph value, alkalinity acts as a buffer, protecting the. Alkalinity is caused by strong bases and the salts of.What is Total Alkalinity? How to Test for TA?
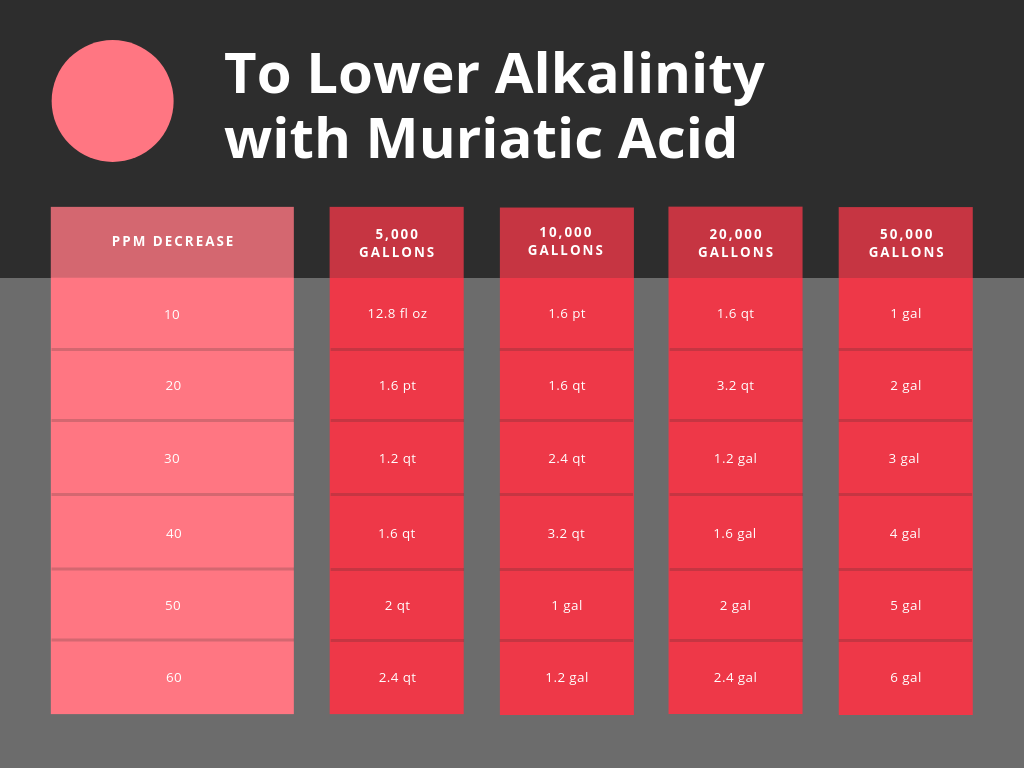
Alkalinity Too High? Here's How to Lower Alkalinity in a Pool Quickly
Poolife™ Alkalinity Plus Alkalinity Increaser for Pools Poolife
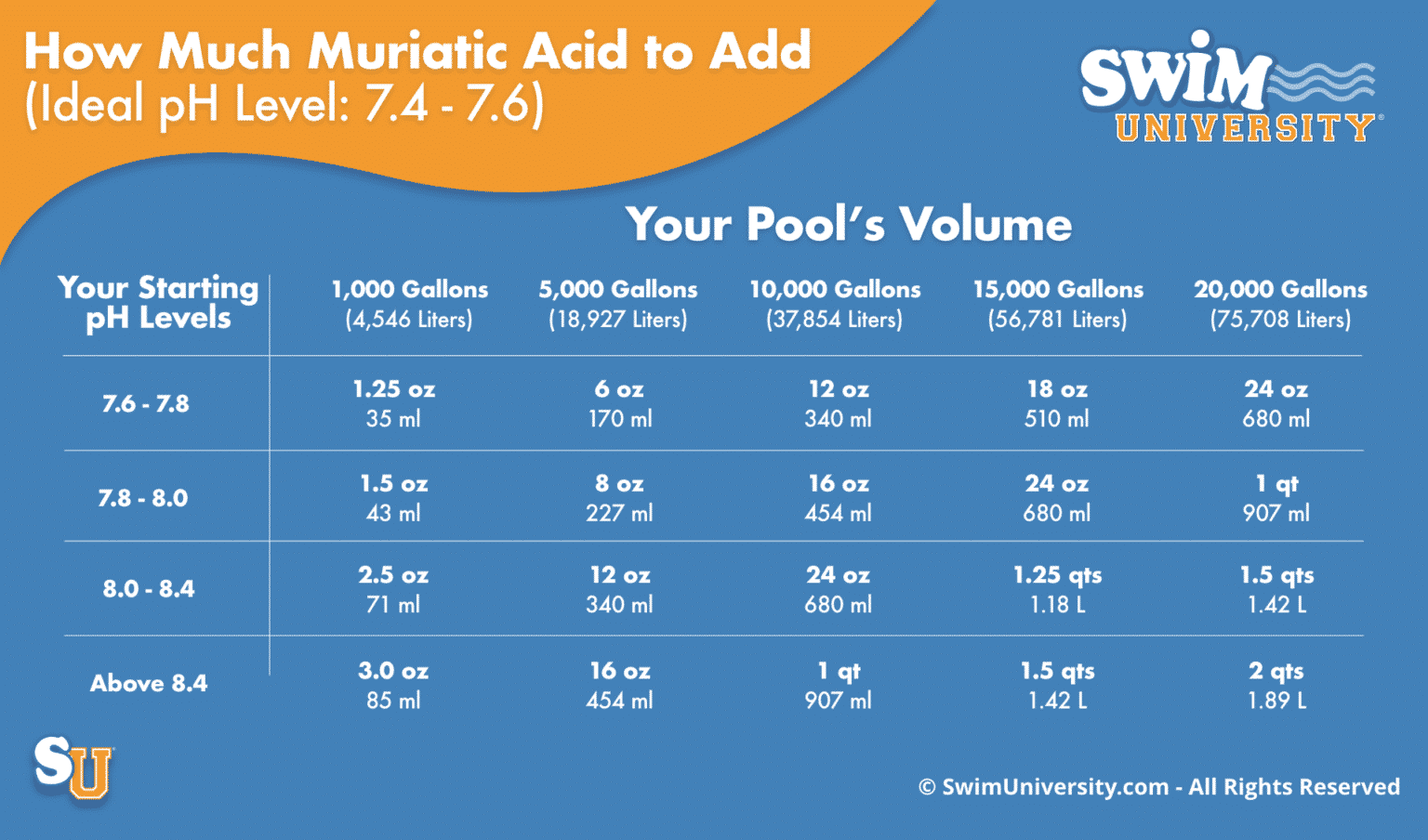
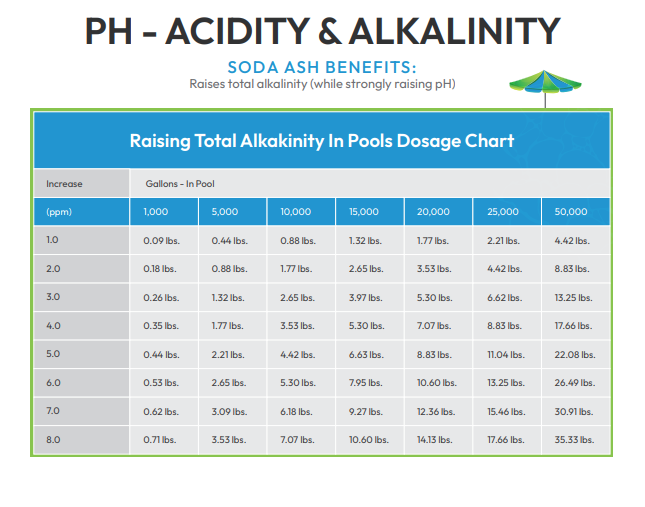
Adjusting pH and Alkalinity in Swimming Pools • Pool Chemistry Training Institute
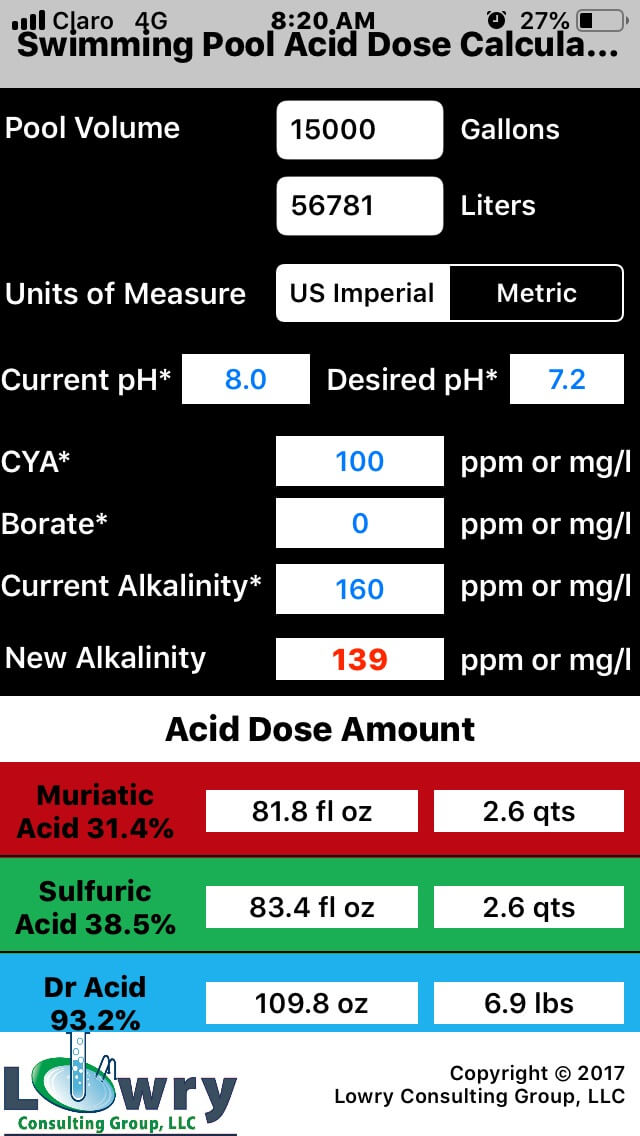
How To Control & Adjust Swimming Pool Alkalinity Doc Deans Pools
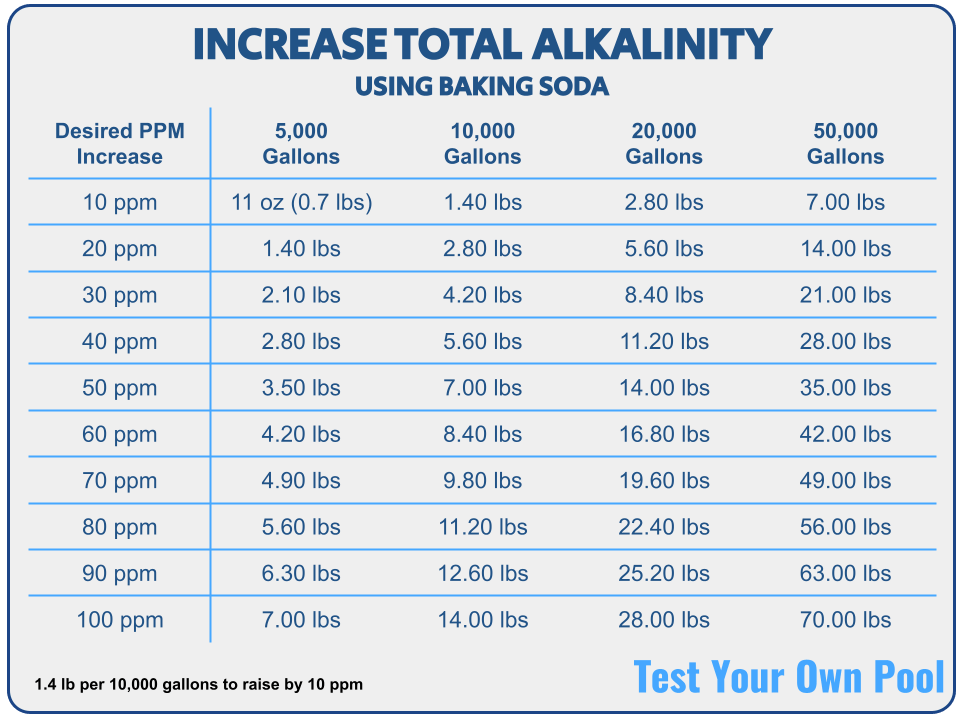
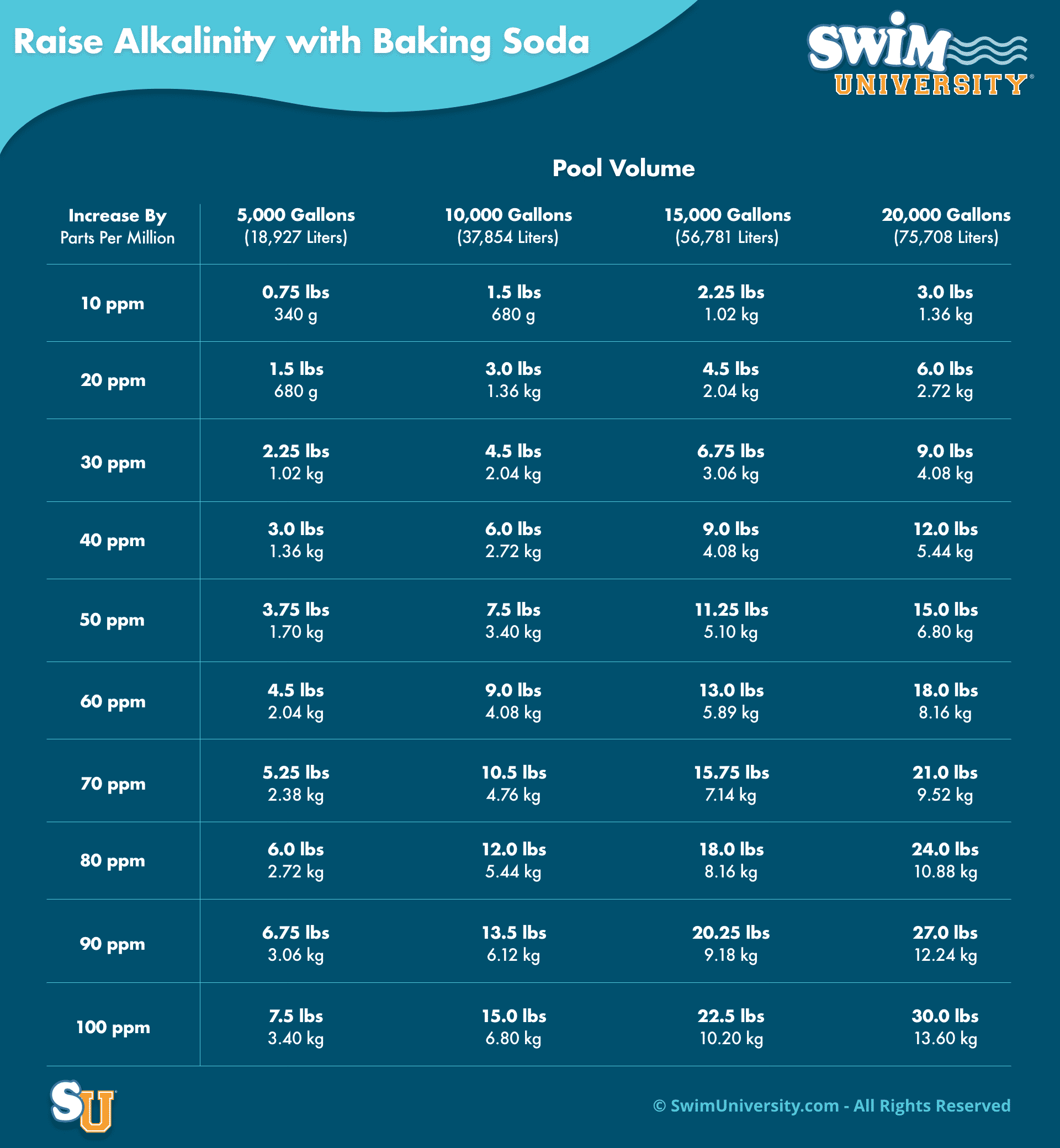
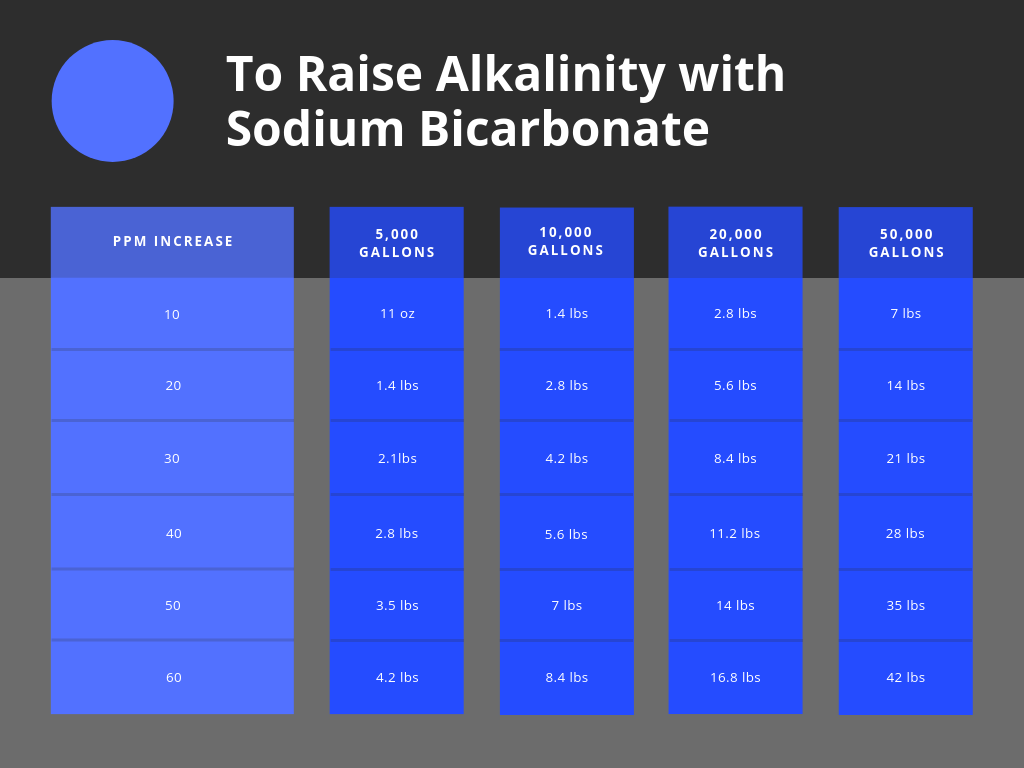
How To Raise Your Pool's Alkalinity with Baking Soda
3 Simple Rules on How To Raise Alkalinity In A Pool Peter Rossi
Alkalinity Chart For Pools Portal.posgradount.edu.pe
How To Control & Adjust Swimming Pool Alkalinity Doc Deans Pools
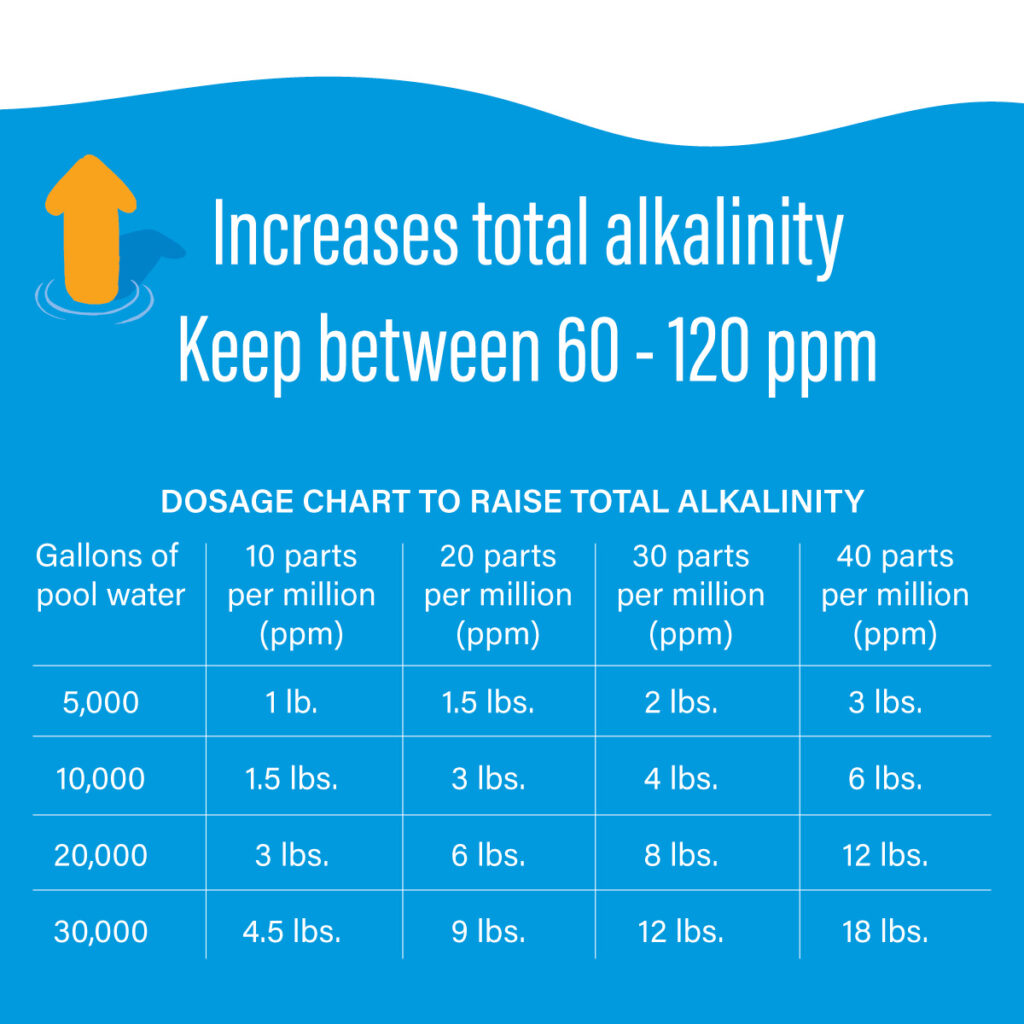
Pool Chemical Basics Water Alkalinity (TA, ALK) Poolsure An Aquasol Company
Alkalinity Measures Water’s Ability To Maintain A Stable Ph Level.
Alkalinity Is Produced By Substances That Yield Hydroxyl Ions Or By Definition, A Substance Is Alkaline If It Will Neutralize Hydrogen Ions.
It Is Measured By Titrating The Solution With An Acid Such As Hcl Until Its Ph Changes Abruptly, Or.
In The World Of Water Chemistry, Alkalinity Often Plays A Crucial, Yet Somewhat Unnoticed, Role.
Related Post: